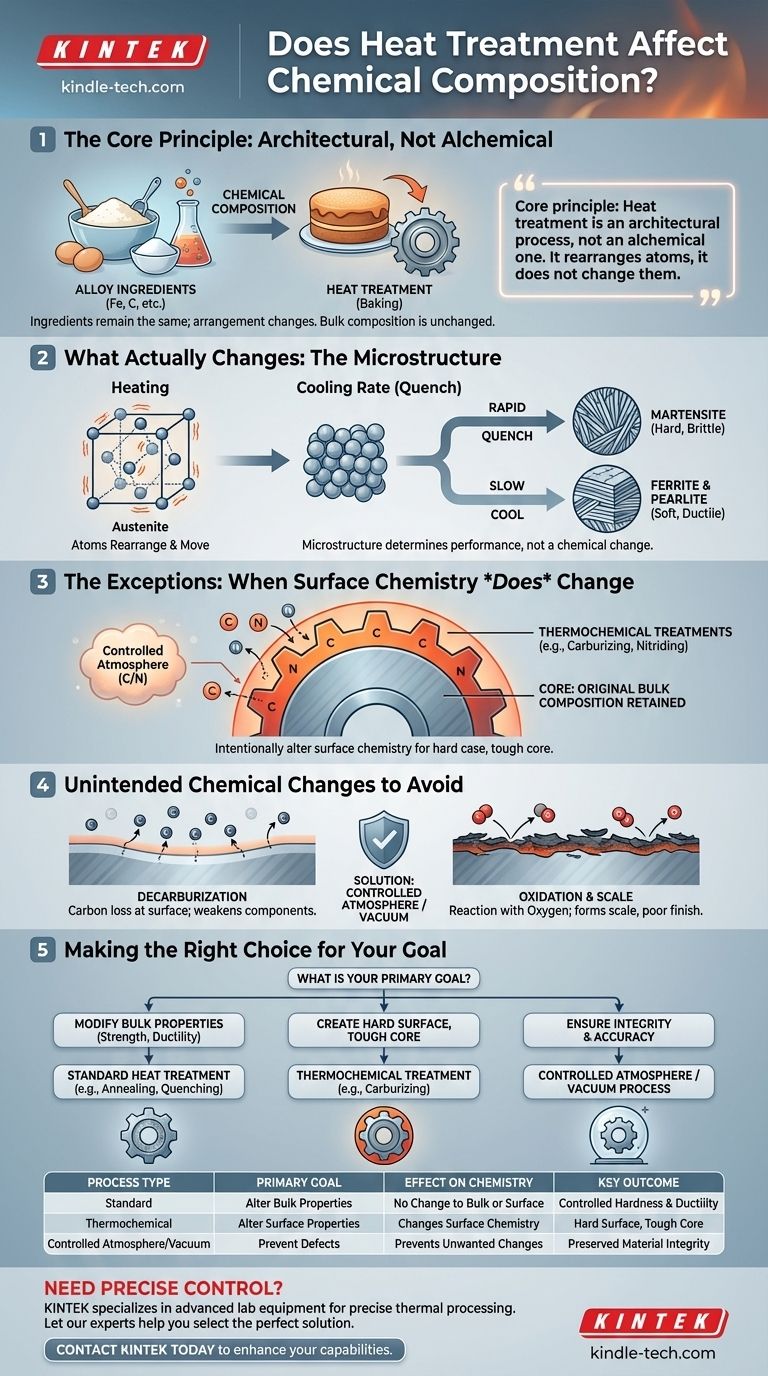
In nearly all standard cases, the answer is no. Conventional heat treatment processes like annealing, quenching, and tempering are designed to alter a metal's physical and mechanical properties—such as hardness and ductility—by changing its internal microstructure, not its fundamental bulk chemical composition. The elemental ingredients of the alloy remain the same throughout its core.
The core principle to understand is that heat treatment is an architectural process, not an alchemical one. It rearranges the existing atoms into different crystal structures to change the material's performance, but it does not change the atoms themselves.

What Heat Treatment Actually Changes: The Microstructure
The true purpose of heat treatment is to manipulate a material's internal structure on a microscopic level. This change in structure is what produces dramatic changes in the final part's behavior.
From Ingredients to Internal Structure
Think of chemical composition as a list of ingredients for a cake—flour, sugar, eggs. These are the elements in the alloy, like iron and carbon in steel.
Heat treatment is the baking process. By controlling temperature and time, you determine the final texture of the cake—whether it's light and fluffy or dense and hard. The ingredients haven't changed, but their arrangement has.
The Role of Crystal Lattices
Metals are crystalline solids, meaning their atoms are arranged in a repeating, orderly pattern called a crystal lattice.
Applying heat gives these atoms the energy to move and rearrange themselves into different lattice structures. For steel, heating it allows the iron and carbon to form a structure called austenite.
The Impact of Cooling Rate
How the metal is cooled (quenched) from this high-temperature state "freezes" a particular structure in place.
A rapid quench traps the carbon atoms, forming a hard, brittle structure called martensite. A slow cool allows atoms to rearrange into softer, more ductile structures like ferrite and pearlite. This is how the same piece of steel can be made either extremely hard or relatively soft without altering its chemical formula.
The Exceptions: When Surface Chemistry Does Change
While the bulk chemistry remains constant, certain specialized processes, known as thermochemical treatments, are designed specifically to alter the chemical composition of the material's surface.
Case Hardening (Carburizing)
Carburizing is a common form of case hardening where a steel part is heated in a carbon-rich atmosphere (like carbon monoxide gas).
This process intentionally forces carbon atoms to diffuse into the surface of the steel, significantly increasing the carbon concentration in the outer layer. The result is a part with a hard, wear-resistant "case" and a softer, tougher core.
Nitriding and Carbonitriding
Similar to carburizing, nitriding introduces nitrogen atoms into the surface of the steel, while carbonitriding introduces both carbon and nitrogen.
These processes create an extremely hard surface layer without the need for the rapid quenching required after carburizing, which reduces the risk of distortion.
The Key Distinction: Bulk vs. Surface
It is critical to remember that in these thermochemical treatments, only the surface chemistry is altered. The core of the material retains its original chemical composition. This dual-property nature is precisely the engineering goal.
Unintended Chemical Changes to Avoid
Sometimes, chemical changes can occur during heat treatment that are undesirable. These are typically the result of the material's surface reacting with the furnace atmosphere at high temperatures.
The Risk of Decarburization
Decarburization is the loss of carbon from the surface of steel. If the furnace atmosphere contains oxygen, it can react with and remove carbon from the part's surface.
This leaves a soft, weakened outer layer, which can be catastrophic for components that rely on surface hardness, like gears or bearings.
Oxidation and Scale Formation
At high temperatures, the metal's surface can react directly with oxygen, forming a dark, flaky layer of metallic oxide known as scale.
This scale represents a loss of base material and results in poor surface finish and dimensional inaccuracy.
The Importance of Atmosphere Control
To prevent these unwanted chemical reactions, modern heat treatment is often performed in controlled atmospheres (like nitrogen or argon) or in a vacuum. This protects the part's surface and ensures that the only changes occurring are the desired microstructural ones.
Making the Right Choice for Your Goal
Selecting the right process depends entirely on whether your goal is to change the entire part uniformly or to create a specialized surface.
- If your primary focus is modifying bulk properties like overall strength, toughness, or ductility: You need a standard heat treatment like annealing, normalizing, quenching, or tempering. These processes will not alter the material's core chemistry.
- If your primary focus is creating a hard, wear-resistant surface while maintaining a tough core: You require a thermochemical treatment like carburizing, nitriding, or carbonitriding, which deliberately changes the surface chemical composition.
- If your primary focus is ensuring material integrity and dimensional accuracy: Your process must include precise atmosphere or vacuum control to prevent unintended and harmful chemical reactions like decarburization and oxidation.
Ultimately, understanding this distinction between rearranging internal structure and altering surface chemistry is the key to mastering material properties through heat treatment.
Summary Table:
| Process Type | Primary Goal | Effect on Chemistry | Key Outcome |
|---|---|---|---|
| Standard (e.g., Annealing, Tempering) | Alter Bulk Properties | No Change to Bulk or Surface | Controlled Hardness & Ductility |
| Thermochemical (e.g., Carburizing) | Alter Surface Properties | Changes Surface Chemistry | Hard Surface, Tough Core |
| Controlled Atmosphere/Vacuum | Prevent Defects | Prevents Unwanted Changes | Preserved Material Integrity |
Need precise control over your material's properties? The right heat treatment process is critical for achieving the perfect balance of hardness, strength, and durability in your components. At KINTEK, we specialize in providing the advanced lab equipment and consumables necessary for precise thermal processing, from standard furnaces to controlled atmosphere systems.
Let our experts help you select the perfect solution for your laboratory's specific needs. Contact KINTEK today to discuss how we can enhance your material testing and processing capabilities.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat and Pressure Sintering Furnace for High Temperature Applications
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What are the advantages of vacuum hardening? Achieve Superior Precision and Cleanliness for Critical Components
- What are vacuum furnaces used for? Unlock Ultimate Material Purity and Performance
- What is the process of a vacuum furnace? Achieve Purity and Precision in High-Temp Processing
- What are the uses of vacuum furnace? Achieve Unmatched Material Purity and Performance
- What is the maximum temperature in a vacuum furnace? It Depends on Your Materials and Process Needs



















