Calculating heat treatment time is not a single formula, but a methodical process based on the material's properties, the part's geometry, and the desired metallurgical outcome. While a common rule of thumb exists, it is merely a starting point. The true calculation involves ensuring the entire part, from surface to core, reaches the target temperature and is held there long enough for the required internal structural changes to occur.
The goal is not merely to heat the metal, but to ensure the entire cross-section—especially the core—reaches and holds a specific temperature for long enough to achieve the desired metallurgical transformation. Time is a function of material, thickness, and the specific process being performed.

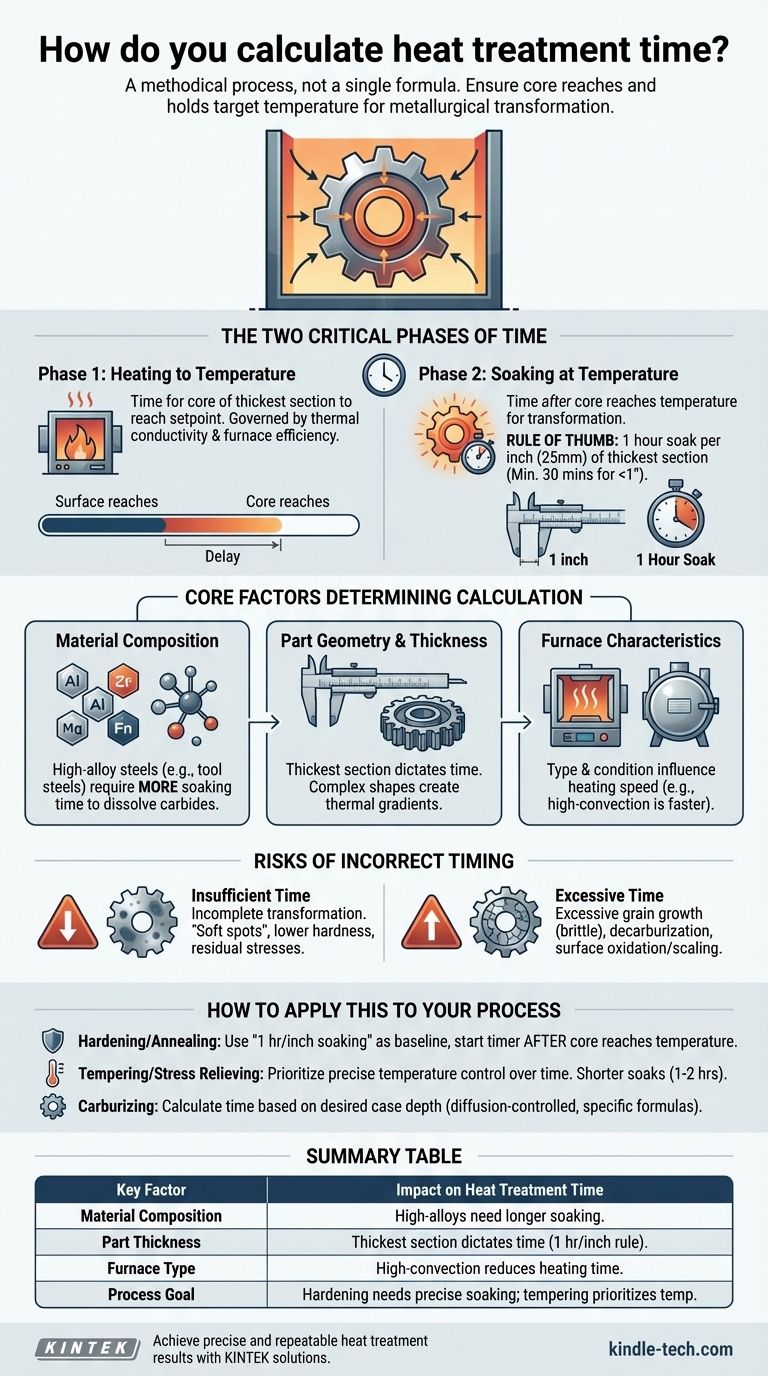
The Two Critical Phases of Time: Heating and Soaking
The total time "at temperature" is misleading. A proper calculation must distinguish between the time it takes to heat the part and the time it is held at the target temperature.
Phase 1: Heating to Temperature
This is the time required for the part to become thermally saturated. The critical factor is ensuring the core of the thickest section reaches the setpoint temperature of the furnace.
This phase is governed by the thermal conductivity of the material and the efficiency of the furnace. A dense pack of parts in an old air furnace will take much longer to heat than a single part in a modern vacuum furnace.
Phase 2: Soaking at Temperature
This is the period after the core has reached temperature. This is the actual "heat treatment time" where the metallurgical magic, such as phase transformation or diffusion, happens.
It is during this phase that a widely used guideline applies: one hour of soaking for every one inch (25 mm) of thickness of the material's thickest cross-section. For parts under one inch, a minimum of 30 minutes is often recommended.
Core Factors That Determine Calculation
A simple rule of thumb is insufficient for critical applications. You must adjust your calculations based on several key variables.
Material Composition
Alloying elements significantly impact transformation time. High-alloy steels (like tool steels) contain elements like chromium, molybdenum, and vanadium that form carbides. These elements require more soaking time to dissolve into the austenite structure compared to a simple plain-carbon steel.
Part Geometry and Thickness
This is the single most dominant factor. Time is always dictated by the thickest section of the part, as it is the last area to reach temperature and complete its transformation. Complex shapes may also create thermal gradients that require careful consideration.
Furnace Characteristics
The type of furnace and its condition directly influence heating time. A high-convection or salt bath furnace provides much faster heat transfer than a static air furnace. The accuracy of furnace thermocouples and the density of the load are also critical variables.
Understanding the Trade-offs: The Risks of Incorrect Timing
Deviating from the optimal time has significant consequences for the final properties of the component.
The Problem with Insufficient Time
If the soaking time is too short, the metallurgical transformation will be incomplete. For hardening, this results in "soft spots" or a part that does not achieve full hardness. For annealing, it can mean residual internal stresses and a structure that is not fully refined.
The Danger of Excessive Time
Holding a part at a high temperature for too long is wasteful and often damaging. The primary risk is excessive grain growth. Large austenitic grains can lead to a coarse, brittle microstructure after quenching, reducing toughness and ductility.
Other risks include decarburization (loss of carbon from the surface, making it soft) and increased surface oxidation or scaling, which may require costly post-processing to remove.
How to Apply This to Your Process
Use the principles above to establish a baseline, but always validate your process for the specific part and equipment.
- If your primary focus is through-hardening or annealing: Use the "1 hour per inch of thickness" rule for soaking time as your starting point, but only begin this timer after you have confirmed the core of the part has reached the target temperature.
- If your primary focus is tempering or stress relieving: Prioritize precise temperature control over time. Soak times are generally shorter (e.g., 1-2 hours) and are less sensitive than for hardening, as the goal is to modify the existing structure, not create a new one.
- If your primary focus is creating a hardened case (carburizing): Calculate time based on the desired case depth. This is a diffusion-controlled process, governed by specific formulas (like Case Depth ≈ K√t, where K is a material/temperature constant and t is time) and requires a different methodology.
Ultimately, a successful heat treatment is the result of methodical testing, validation, and a deep understanding of your specific material and equipment.
Summary Table:
| Key Factor | Impact on Heat Treatment Time |
|---|---|
| Material Composition | High-alloy steels require longer soaking times for complete transformation. |
| Part Thickness | Time is dictated by the thickest section; use the 1 hour per inch rule as a baseline. |
| Furnace Type | High-convection or salt bath furnaces reduce heating time compared to static air furnaces. |
| Process Goal | Hardening requires precise soaking; tempering prioritizes temperature control over time. |
Achieve precise and repeatable heat treatment results with KINTEK.
Our expertise in lab equipment and consumables ensures you have the right tools for accurate temperature control and process validation. Whether you're working with high-alloy steels or complex geometries, KINTEK provides reliable solutions to optimize your heat treatment cycles and avoid costly errors like soft spots or excessive grain growth.
Contact us today to discuss how our products can enhance your laboratory's efficiency and ensure your heat treatment processes deliver consistent, high-quality outcomes.
Visual Guide
Related Products
- Vertical Laboratory Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
People Also Ask
- How do you clean a quartz tube furnace? Prevent Contamination & Extend Tube Lifespan
- What is a vertical tube furnace? Leverage Gravity for Superior Uniformity and Process Control
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost
- What is the process of annealing tubes? Achieve Optimal Softness and Ductility for Your Tubing
- What is the difference between upflow and horizontal furnace? Find the Perfect Fit for Your Home's Layout



















