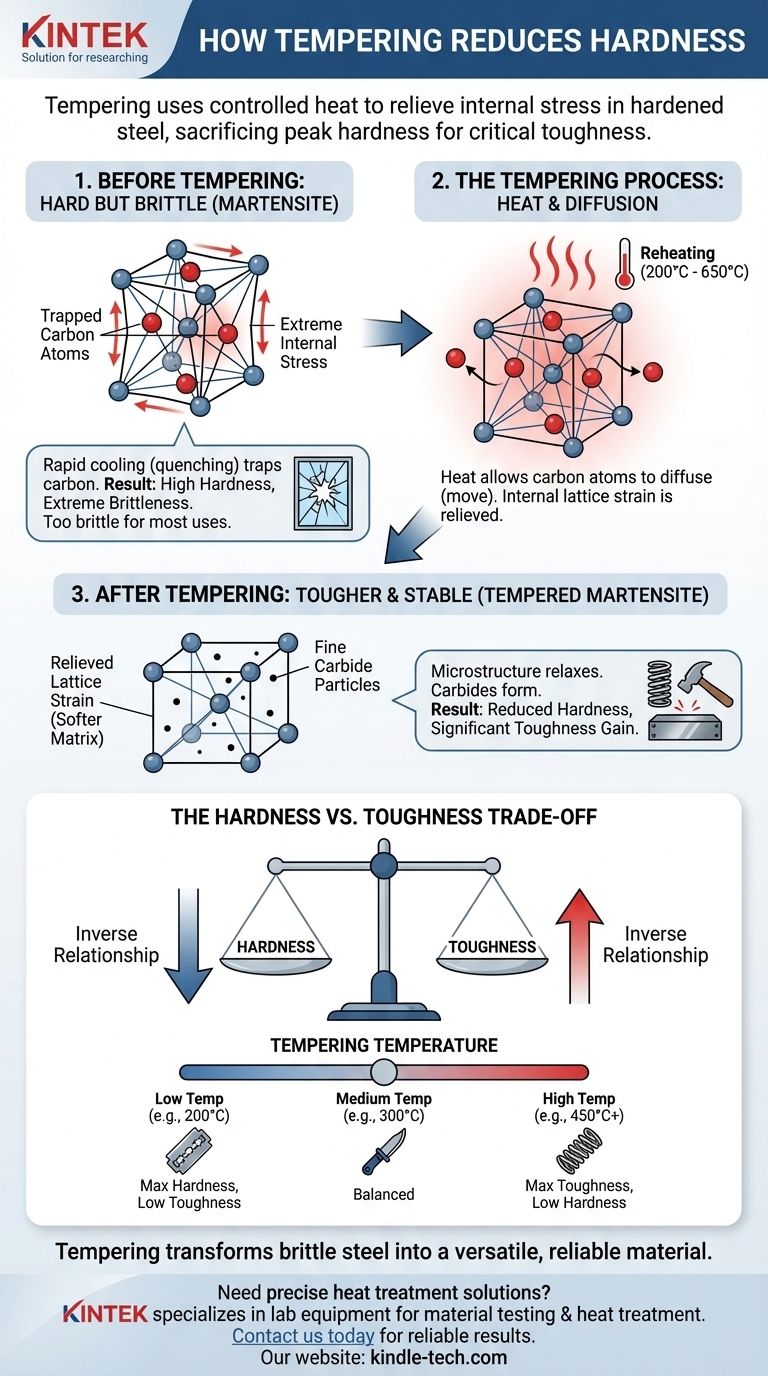
At its core, tempering reduces hardness by providing just enough heat to relieve the immense internal stress within hardened steel. This controlled reheating allows trapped carbon atoms to move, transforming the steel's brittle, highly-strained microstructure into a tougher, more stable one. While the primary goal is a massive gain in toughness, a predictable reduction in hardness is the direct consequence.
Untempered steel, known as martensite, is extremely hard but too brittle for almost any practical use. Tempering is the essential process of sacrificing a controlled amount of that peak hardness to gain the critical toughness required for a durable and reliable component.
The State of Steel Before Tempering: Hard but Brittle
To understand tempering, you must first understand the material it acts upon. Steel is typically tempered immediately after a hardening process called quenching.
The Quenching Process and Martensite
During hardening, steel is heated to a high temperature where its crystal structure becomes austenite, a form that can dissolve a significant amount of carbon. When this hot steel is cooled rapidly (quenched), the carbon atoms are trapped within the iron crystal lattice.
This rapid cooling forces the austenite to transform into martensite, a body-centered tetragonal (BCT) crystal structure.
Why Martensite is So Hard
The defining characteristic of martensite is its extreme internal stress. The trapped carbon atoms distort the iron crystal lattice, preventing the crystal planes from slipping past one another.
This resistance to slip, known as dislocation movement, is the very definition of hardness at a microscopic level. The more stress, the harder the material.
The Critical Flaw: Extreme Brittleness
This high hardness comes at a severe cost: extreme brittleness. The immense internal stress makes the material behave like glass. It has very low resistance to fracture and will shatter under sharp impact or bending.
For tools like knives, axes, or structural parts like bolts, this brittleness is a catastrophic failure point.
The Mechanics of Tempering: Relieving Internal Stress
Tempering is a sub-critical heat treatment, meaning the steel is reheated to a temperature below the point where it would transform back to austenite (typically between 200°C and 650°C / 400°F and 1200°F).
The Role of Heat and Carbon Diffusion
The added thermal energy from reheating allows the "frozen" carbon atoms to finally move, or diffuse, through the crystal lattice. This is the central mechanism of tempering.
From Strained Martensite to a Softer Matrix
As carbon atoms migrate out of their trapped positions, the severe distortion of the iron crystal lattice is relieved. The highly-strained BCT martensite structure relaxes into a much less-strained body-centered cubic (BCC) structure, which is essentially a form of ferrite.
This ferrite matrix is inherently softer than the original martensite because the primary source of its hardness—the lattice strain—has been significantly reduced.
The Formation of Fine Carbides
The migrating carbon atoms don't just disappear. They combine with iron atoms to form new, microscopic particles of iron carbide (Fe₃C), also known as cementite.
The final structure, called tempered martensite, is a composite material: a softer ferrite matrix reinforced by a fine dispersion of very hard carbide particles. While these carbides add some hardness, the overall effect of relieving the internal lattice stress is a net decrease in the steel's total hardness.
Understanding the Trade-offs: Hardness vs. Toughness
The relationship between hardness and toughness is the most important concept in heat treatment. Tempering is the process of deliberately navigating this trade-off.
The Inverse Relationship
For a given steel, hardness and toughness are generally inversely proportional. As you temper the steel to decrease its hardness, you will almost always increase its toughness, which is the material's ability to absorb energy and deform without fracturing.
Controlling the Outcome with Temperature
The tempering temperature is the primary control variable. A higher tempering temperature provides more thermal energy, allowing for more carbon diffusion, more stress relief, and the formation of larger carbide particles.
- Lower Temperature: Results in a small drop in hardness and a significant gain in toughness.
- Higher Temperature: Results in a larger drop in hardness and a maximum gain in toughness.
Making the Right Choice for Your Goal
The ideal balance of hardness and toughness is dictated entirely by the tool's intended application. The tempering temperature is chosen specifically to achieve this balance.
- If your primary focus is extreme wear resistance and edge holding (e.g., razor blades, files): Use a low tempering temperature (around 200°C / 400°F) to retain maximum hardness while relieving just enough stress to prevent chipping.
- If your primary focus is a balance of hardness and impact resistance (e.g., knives, chisels, axe heads): Use a medium tempering temperature (260-340°C / 500-650°F) to achieve good edge retention and the toughness to withstand moderate impact.
- If your primary focus is maximum toughness and flexibility (e.g., springs, structural bolts, swords): Use a high tempering temperature (450°C+ / 850°F+) to sacrifice significant hardness for the ability to bend, flex, and absorb severe shock without breaking.
Tempering is what transforms steel from a brittle curiosity into the versatile and reliable foundation of the modern world.

Summary Table:
| Tempering Temperature | Effect on Hardness | Effect on Toughness | Ideal Application |
|---|---|---|---|
| Low (~200°C / 400°F) | Small decrease | Significant increase | Razor blades, files |
| Medium (260-340°C / 500-650°F) | Moderate decrease | High increase | Knives, chisels, axes |
| High (450°C+ / 850°F+) | Large decrease | Maximum increase | Springs, swords, bolts |
Need precise heat treatment for your steel components? KINTEK specializes in laboratory equipment and consumables for material testing and heat treatment processes. Our expertise ensures you achieve the exact hardness-toughness balance required for your specific application. Contact us today to discuss how our solutions can enhance your lab's capabilities and deliver reliable, durable results.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What function does a high-temperature annealing furnace serve in tungsten oxide post-processing? Key Phase Control
- How do you check the temperature of a muffle furnace? A Guide to Precise Monitoring
- How is a benchtop laboratory oven utilized in processing spongin-atacamite composites? Achieve Precise Material Drying
- What role does a high-temperature muffle furnace play in HE-O-MIEC synthesis? Achieve Precision Ceramic Engineering
- Why is a high-temperature furnace with precision control required for HAp synthesis? Ensure Medical-Grade Purity
- Why is a high-temperature furnace essential for catalyst preparation? Unlock peak catalytic activity and stability.
- What are the applications of sintering? Unlock High-Strength, Complex Parts Manufacturing
- How is a kiln different from an oven? Understanding Heat, Purpose, and Material Transformation



















