In short, Chemical Vapor Deposition (CVD) synthesizes graphene by flowing a carbon-containing gas over a hot metal catalyst, typically a copper foil. The high temperature breaks the gas down, depositing individual carbon atoms onto the metal surface. These atoms then self-assemble into a continuous, single-atom-thick sheet of graphene that blankets the catalyst.
The core challenge of making graphene isn't just creating carbon, but arranging it into a perfect, large-scale, one-atom-thick lattice. CVD solves this by using a heated metal catalyst as an atomic-scale template, guiding the assembly of carbon atoms from a simple gas into a highly ordered and uniform 2D crystal.

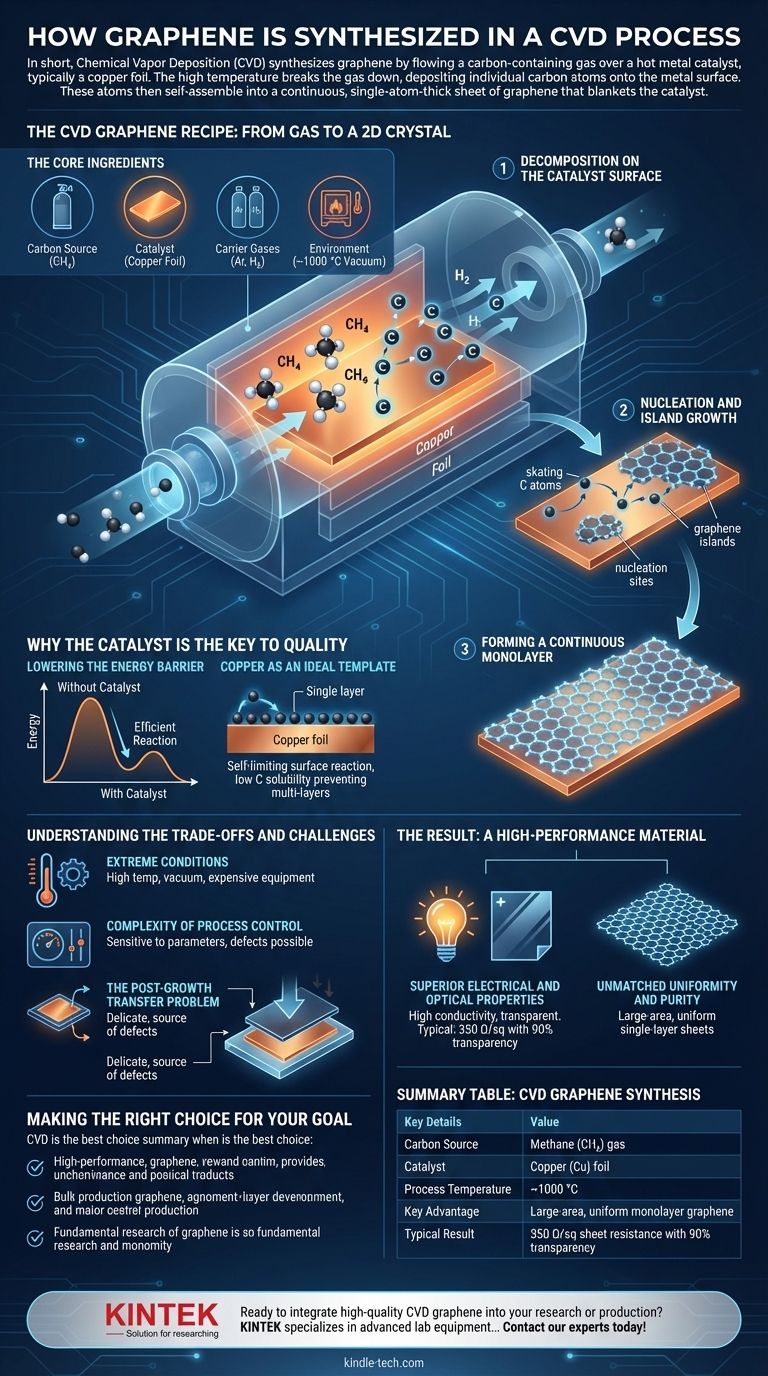
The CVD Graphene Recipe: From Gas to a 2D Crystal
The CVD process can be understood as a highly controlled, high-temperature chemical reaction. It requires a specific set of ingredients and a precise sequence of events to achieve a high-quality result.
The Core Ingredients
The process takes place inside a vacuum-sealed furnace. The key components are:
- Carbon Source: A hydrocarbon gas, most commonly methane (CH₄).
- Catalyst: A metal foil that provides the surface for growth. Copper (Cu) is widely used for monolayer graphene.
- Carrier Gases: Inert gases like Argon (Ar) and Hydrogen (H₂) are used to control the pressure and clean the catalyst surface.
- Environment: A high-temperature furnace capable of reaching around 1000 °C.
Step 1: Decomposition on the Catalyst Surface
The process begins by heating the copper foil in the furnace under a flow of hydrogen gas to clean its surface. Once the target temperature is reached, a small amount of methane is introduced into the chamber.
The intense heat causes the methane molecules to break apart, a process known as pyrolysis. This frees carbon atoms, which then deposit onto the hot surface of the copper catalyst.
Step 2: Nucleation and Island Growth
The individual carbon atoms are not static. They diffuse, or "skate," across the catalyst surface.
Eventually, these mobile atoms collide and bond, forming tiny, stable patches of graphene. These initial patches are called nucleation sites. From these sites, the graphene begins to grow outwards in hexagonal "islands."
Step 3: Forming a Continuous Monolayer
As the process continues, more carbon atoms attach to the edges of these growing islands. The islands expand across the copper surface until their edges meet.
They then stitch together, forming a single, continuous, and largely seamless sheet of monolayer graphene that covers the entire surface of the copper foil.
Why the Catalyst is the Key to Quality
The metal substrate is not merely a passive surface; it is an active and essential catalyst that dictates the outcome of the entire process.
Lowering the Energy Barrier
Without a catalyst, decomposing methane to form a perfect graphene sheet would require far higher energy and temperature. The catalyst lowers the activation energy for the reaction, making the process more efficient and controlled.
Copper as an Ideal Template
Copper is the preferred catalyst for single-layer graphene due to its very low carbon solubility. This means carbon atoms do not dissolve into the copper. Instead, they remain on the surface.
This surface-limited reaction is self-limiting: once the copper is covered by a complete layer of graphene, the catalytic process stops, preventing the formation of unwanted additional layers.
Understanding the Trade-offs and Challenges
While CVD is considered the best method for producing high-quality graphene, it is not without its complexities and limitations.
The Need for Extreme Conditions
The process requires very high temperatures (around 1000 °C) and a vacuum environment. This necessitates specialized, energy-intensive equipment and makes the process relatively expensive.
Complexity of Process Control
The final quality of the graphene is extremely sensitive to process parameters. Gas flow rates, temperature stability, and pressure must be controlled with high precision. Any deviation can introduce defects, wrinkles, or multiple layers into the graphene sheet.
The Post-Growth Transfer Problem
Graphene grown on a metal foil is rarely used there. It must be transferred to a target substrate (like silicon or glass). This delicate process typically involves coating the graphene with a polymer, etching away the metal catalyst, and then "stamping" the graphene onto its new substrate. This transfer step is a major source of defects, tears, and contamination.
The Result: A High-Performance Material
Despite the challenges, the results of a well-executed CVD process are unparalleled, producing graphene with properties ideal for next-generation technology.
Superior Electrical and Optical Properties
CVD graphene exhibits an excellent combination of high electrical conductivity and optical transparency. For example, a sheet resistance of 350 Ω/sq with 90% transparency is a typical benchmark, making it a prime candidate for use as a transparent conductive film in touch screens, flexible electronics, and solar cells.
Unmatched Uniformity and Purity
The primary advantage of CVD is its ability to produce large-area films with high homogeneity and purity. The precise control over the growth mechanism allows for the creation of uniform single-layer sheets, a feat that is difficult to achieve with other synthesis methods.
Making the Right Choice for Your Goal
Understanding the principles of CVD allows you to determine if it aligns with your specific technical or commercial objectives.
- If your primary focus is high-performance electronics or optics: CVD is the industry-standard method for producing the required high-quality, uniform monolayer graphene.
- If your primary focus is bulk production for composites or inks: Other methods like liquid-phase exfoliation may be more cost-effective, as the pristine quality and uniformity of CVD are often unnecessary for these applications.
- If your primary focus is fundamental research on growth mechanisms: The CVD platform is highly tunable, offering an ideal environment to study the physics of 2D material formation by systematically varying catalysts, precursors, and conditions.
Mastering CVD is about leveraging chemistry and thermodynamics to engineer a material at the atomic scale, transforming a simple gas into a revolutionary material.
Summary Table:
| CVD Graphene Synthesis | Key Details |
|---|---|
| Carbon Source | Methane (CH₄) gas |
| Catalyst | Copper (Cu) foil |
| Process Temperature | ~1000 °C |
| Key Advantage | Large-area, uniform monolayer graphene |
| Typical Result | 350 Ω/sq sheet resistance with 90% transparency |
Ready to integrate high-quality CVD graphene into your research or production? KINTEK specializes in providing the advanced lab equipment and consumables needed for precise graphene synthesis. Our expertise in furnaces, gas delivery systems, and process control can help you achieve superior material properties and accelerate your development timeline. Contact our experts today to discuss your specific graphene synthesis requirements!
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What is PECVD in semiconductor? Enable Low-Temperature Thin Film Deposition for ICs
- What is the process of vacuum vapor deposition? Mastering CVD and PVD Thin-Film Coating
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- What color diamonds are CVD? Understanding the Process from Brown Tint to Colorless Beauty
- What are the methods of deposition? A Guide to PVD and CVD Thin-Film Techniques



















