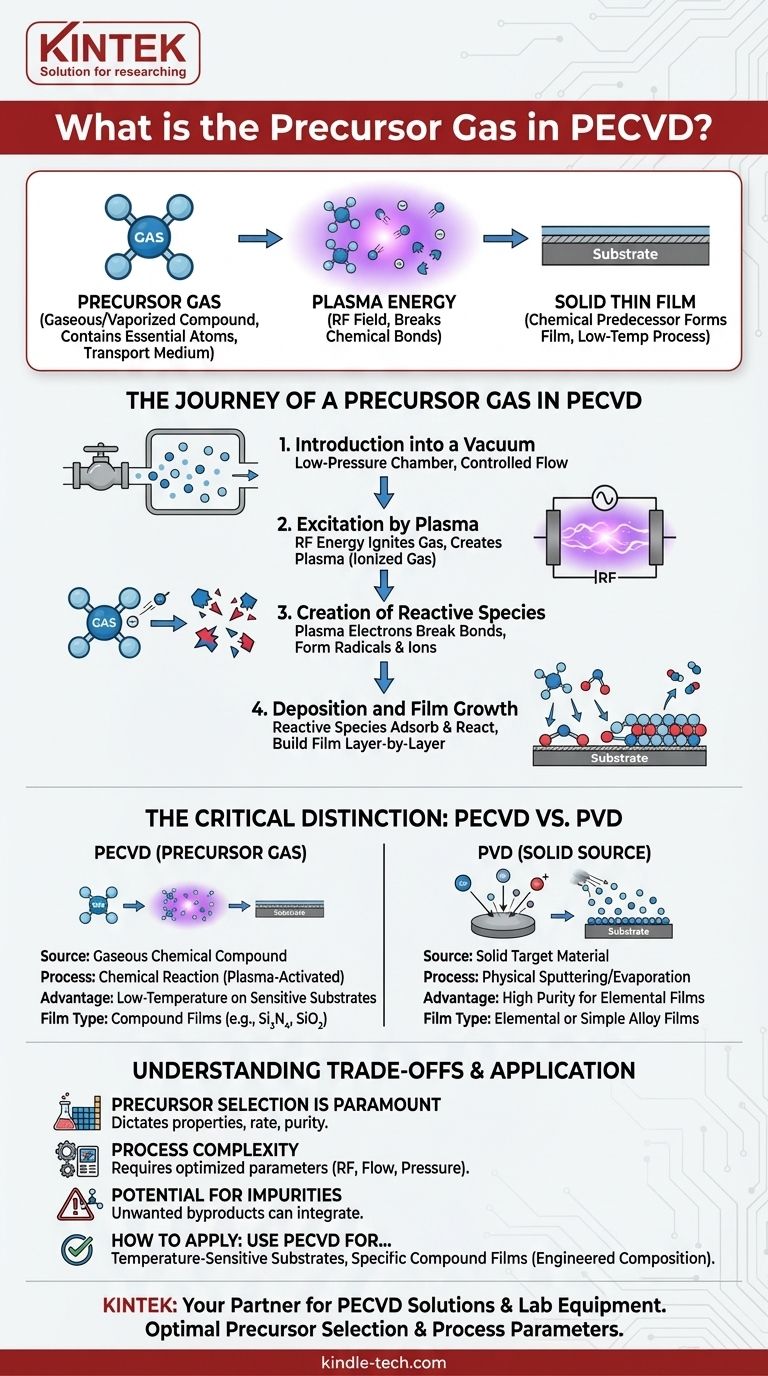
In Plasma Enhanced Chemical Vapor Deposition (PECVD), the precursor gas is the specific gaseous or vaporized chemical compound introduced into the reaction chamber. This gas contains the essential atoms that will ultimately form the solid thin film on a substrate. It serves as the raw material, or chemical predecessor, which is broken down by plasma to initiate the deposition process.
The core function of a precursor gas is to act as a transport medium, delivering the desired elements to the substrate in a stable, gaseous form. The innovation of PECVD is its use of plasma energy—not just high heat—to break apart these stable precursor molecules, enabling high-quality film deposition at much lower temperatures.

The Journey of a Precursor Gas in PECVD
To fully grasp the concept, it's essential to understand the step-by-step role the precursor plays from its introduction into the chamber to its final transformation into a solid film.
Step 1: Introduction into a Vacuum
A precisely controlled flow of one or more precursor gases is introduced into a low-pressure vacuum chamber. The choice of gas is critical, as it directly determines the chemical composition of the final film.
Step 2: Excitation by Plasma
An electrical field, typically Radio Frequency (RF), is applied across electrodes in the chamber. This energy ignites the precursor gas, stripping electrons from some of the gas molecules and creating a plasma.
This plasma is a highly energetic, ionized gas containing a mix of neutral molecules, free radicals, ions, and high-energy electrons.
Step 3: Creation of Reactive Species
The high-energy electrons within the plasma collide with the stable precursor gas molecules. These collisions transfer energy, breaking the chemical bonds of the precursor.
This is the key step that distinguishes PECVD. Instead of relying on high thermal energy (heat) to break bonds, it uses plasma energy. This creates highly reactive chemical fragments, known as radicals and ions.
Step 4: Deposition and Film Growth
These newly formed, highly reactive species diffuse through the chamber and reach the surface of the substrate.
Upon arrival, they readily react with the surface and with each other, a process called adsorption. As they bond to the surface, they build the desired solid thin film, layer by layer. Unwanted chemical byproducts are removed from the chamber by the vacuum system.
The Critical Distinction from Other Methods
Understanding what makes a "precursor gas" unique to this process clarifies why PECVD is used for specific applications.
A Chemical Predecessor, Not a Physical Source
The term "precursor" literally means "forerunner" or "predecessor." The gas itself is not the final material. It is a stable compound that undergoes a chemical reaction to become the film.
For example, to deposit a silicon nitride (Si₃N₄) film, one might use silane (SiH₄) and ammonia (NH₃) as precursor gases. The plasma breaks them down, allowing silicon and nitrogen atoms to recombine on the substrate.
The Key Difference from PVD
This chemical process is fundamentally different from Physical Vapor Deposition (PVD).
In PVD, the source material is a solid target. Energy is used to physically knock atoms off this target (sputtering) or boil them off (evaporation), which then travel and coat the substrate. There is no intended chemical reaction.
In PECVD, the source material is a gas that is chemically transformed to create the film.
Understanding the Trade-offs
While powerful, the use of chemical precursors in a plasma environment comes with specific considerations.
Precursor Selection is Paramount
The choice of precursor gas dictates the film's properties, deposition rate, and purity. Some precursors are more effective but can be more hazardous, expensive, or difficult to handle than others.
Process Complexity
Controlling a plasma-based chemical reaction is more complex than a purely thermal or physical process. Factors like RF power, gas flow rates, pressure, and chamber geometry must be meticulously optimized to achieve a uniform, high-quality film.
Potential for Impurities
Because PECVD is a chemical reaction, unwanted byproducts can sometimes be incorporated into the film as impurities if the process parameters are not perfectly controlled. For instance, hydrogen from a precursor like silane (SiH₄) can remain in a deposited silicon film.
How to Apply This to Your Project
Your deposition strategy should be guided by your material requirements and substrate limitations.
- If your primary focus is depositing on temperature-sensitive substrates: PECVD is the superior choice, as the plasma provides the reaction energy without requiring destructive high heat.
- If your primary focus is depositing a pure, elemental film from a solid source: PVD is often a more direct and cleaner method, as it avoids the complexities of gas-phase chemical reactions.
- If your primary focus is creating a specific compound film (e.g., silicon dioxide, silicon nitride): PECVD offers exceptional control by allowing you to mix different precursor gases to precisely engineer the film's chemical composition.
Understanding that the precursor gas is a reactive ingredient, not just a physical source, is the key to mastering the PECVD process and its unique capabilities.
Summary Table:
| Aspect | PECVD Precursor Gas | PVD Solid Source |
|---|---|---|
| Source Form | Gaseous or vaporized chemical compound | Solid target material |
| Process Type | Chemical reaction (plasma-activated) | Physical sputtering/evaporation |
| Key Advantage | Low-temperature deposition on sensitive substrates | High purity for elemental films |
| Film Type | Compound films (e.g., Si₃N₄, SiO₂) | Elemental or simple alloy films |
Need to deposit high-quality thin films on temperature-sensitive substrates? KINTEK specializes in PECVD systems and lab equipment, offering tailored solutions for your precise material requirements. Our expertise ensures optimal precursor selection and process parameters for superior film quality and performance. Contact our experts today to discuss how our PECVD solutions can enhance your research or production process.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Multi-zone Laboratory Tube Furnace
People Also Ask
- What is the significance of good conformal step coverage provided by PECVD? Ensure Device Integrity and Reliability
- What is the frequency of PECVD? Mastering Plasma Control for Superior Thin Films
- How does plasma enhance CVD? Unlock Low-Temperature, High-Quality Film Deposition
- How do PECVD systems improve DLC coatings on implants? Superior Durability and Biocompatibility Explained
- What temperature is DLC application? Achieve Superior Coatings Without Damaging Your Parts
- How does post-treatment in an annealing furnace improve PECVD a-SiC thin films? Achieve Superior Material Stability
- What is plasma assisted physical vapor deposition? Enhance Your Coating Performance with Advanced PA-PVD
- What are the advantages of using a 316 stainless steel cathodic cage? Enhance Precision in Plasma Nitriding



















