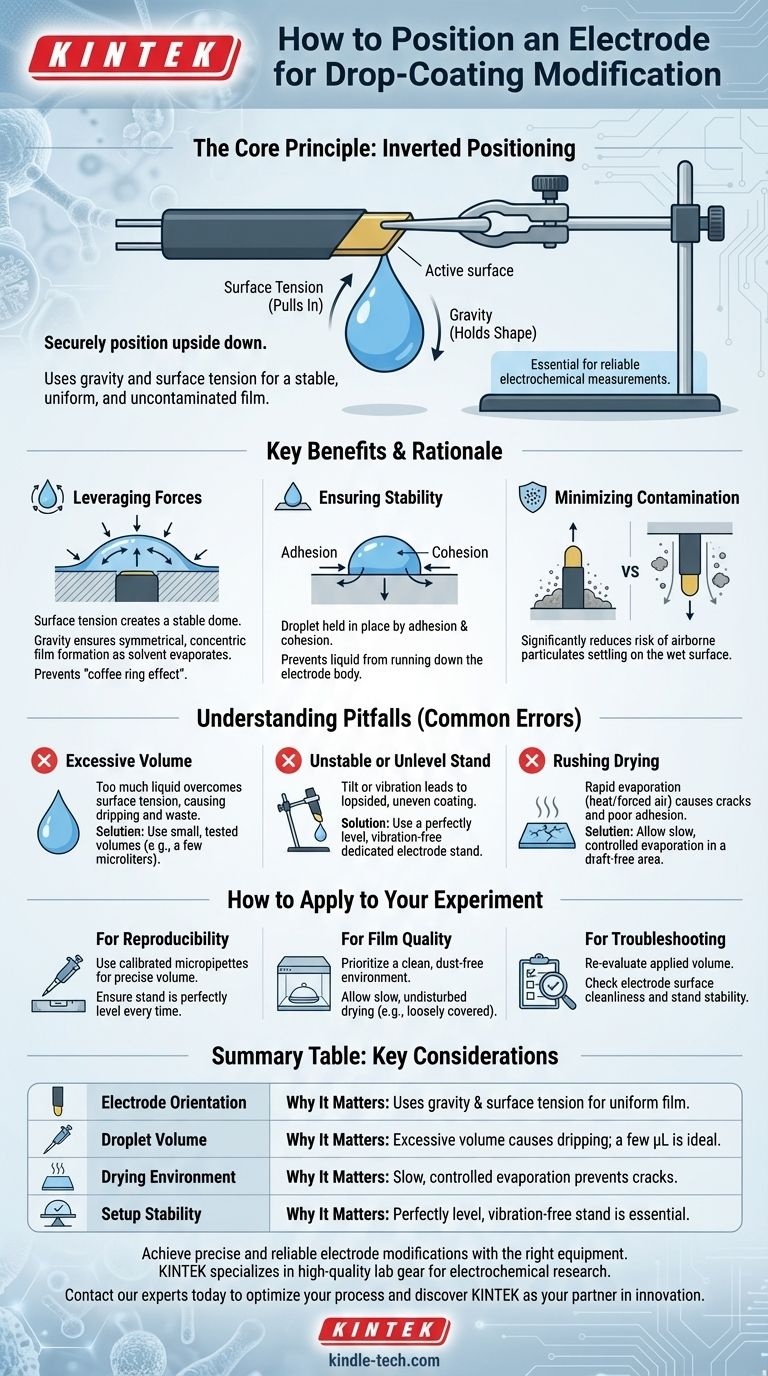
To properly modify an electrode via drop-coating, it should be securely positioned upside down. This orientation is essential for using gravity and surface tension to form a stable, uniform, and uncontaminated film on the electrode's active surface.
The core principle is simple yet critical: an inverted electrode allows the liquid droplet to hang symmetrically, held by surface tension. This promotes even drying and creates a consistent coating, which is the foundation for reliable electrochemical measurements.

The Rationale Behind the "Upside-Down" Method
Understanding why this specific orientation is standard practice is key to achieving reproducible and high-quality results. The method is designed to control physical forces to your advantage.
Leveraging Gravity and Surface Tension
When a small droplet of coating material is applied to the inverted electrode surface, surface tension pulls the liquid into a stable, dome-like shape.
Gravity helps hold this symmetrical shape, ensuring that as the solvent evaporates, the deposited material forms a uniform and concentric film.
This prevents the common issue of the "coffee ring effect," where material accumulates unevenly at the edges of the droplet.
Ensuring Droplet Stability
Positioning the electrode upside down prevents the modification liquid from running down the sides of the electrode body.
The droplet is held securely in place by the forces of adhesion (to the electrode surface) and cohesion (within the liquid itself), so long as the volume is not excessive.
This stability is crucial for ensuring the entire measured volume of the coating material contributes to the final film on the active area.
Minimizing Airborne Contamination
An upward-facing surface acts as a natural collection point for dust and other airborne particulates.
By inverting the electrode, you significantly reduce the risk of contaminants settling onto the wet surface during the application and drying phases. This is a simple and effective form of contamination control.
Understanding the Trade-offs and Pitfalls
While the inverted method is standard, its success depends on careful execution. A few common errors can compromise the entire process.
Applying Excessive Volume
The most frequent mistake is using a droplet volume that is too large for the electrode's surface area.
If the droplet's weight exceeds the force of surface tension, it will simply drip off, wasting valuable material and resulting in a failed coating. Always start with a small, tested volume (typically a few microliters).
Using an Unstable or Unlevel Stand
The electrode must be held perfectly level in a dedicated electrode stand.
Any slight tilt will cause the droplet to shift, leading to an uneven, lopsided coating upon drying. Vibrations can also disturb the droplet, so work on a stable bench.
Rushing the Drying Process
Proper film formation requires slow, controlled evaporation of the solvent.
Placing the setup in a draft-free location, sometimes loosely covered (e.g., with a petri dish), allows the solvent to evaporate gently. Rushing this step with heat or forced air can cause cracks and poor adhesion in the final film.
How to Apply This to Your Experiment
Your specific goal will determine which procedural details to emphasize for a successful modification.
- If your primary focus is reproducibility: Use a calibrated micropipette for precise volume control and ensure your electrode stand is perfectly level every single time.
- If your primary focus is film quality: Prioritize a clean, dust-free environment and allow for a slow, undisturbed drying process to create a uniform, well-adhered layer.
- If you are troubleshooting inconsistent results: Re-evaluate your applied volume, check the cleanliness of your electrode surface before coating, and confirm your stand is completely stable.
Mastering this fundamental technique is a crucial step toward achieving reliable and accurate electrochemical data.
Summary Table:
| Key Consideration | Why It Matters |
|---|---|
| Electrode Orientation | Upside-down positioning uses gravity and surface tension for a uniform film. |
| Droplet Volume | Excessive volume causes dripping; a few microliters is typically ideal. |
| Drying Environment | Slow, controlled evaporation in a draft-free area prevents cracks. |
| Setup Stability | A perfectly level, vibration-free stand is essential for an even coating. |
Achieve precise and reliable electrode modifications with the right equipment.
KINTEK specializes in high-quality lab equipment and consumables for all your electrochemical research needs. From stable electrode stands to precise micropipettes, our products are designed to support reproducible results and enhance your lab's efficiency.
Let us help you optimize your process. Contact our experts today to discuss your specific application and discover how KINTEK can be your partner in innovation.
Visual Guide
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Electrochemical Sheet Electrode Gold Electrode
- Gold Disc Electrode
- Metal Disc Electrode Electrochemical Electrode
People Also Ask
- Why is a Saturated Calomel Electrode (SCE) used as a reference electrode in microbial fuel cell research?
- Why is the calomel electrode used as a secondary reference electrode? A Practical Guide to Stable Measurements
- Which electrode is used as a reference? A Guide to Accurate Electrochemical Measurements
- What are the characteristics of a saturated calomel electrode for neutral solutions? Understanding its stability and limitations.
- What is the reference electrode for mercury mercurous sulfate? A Guide to Chloride-Free Electrochemistry



















