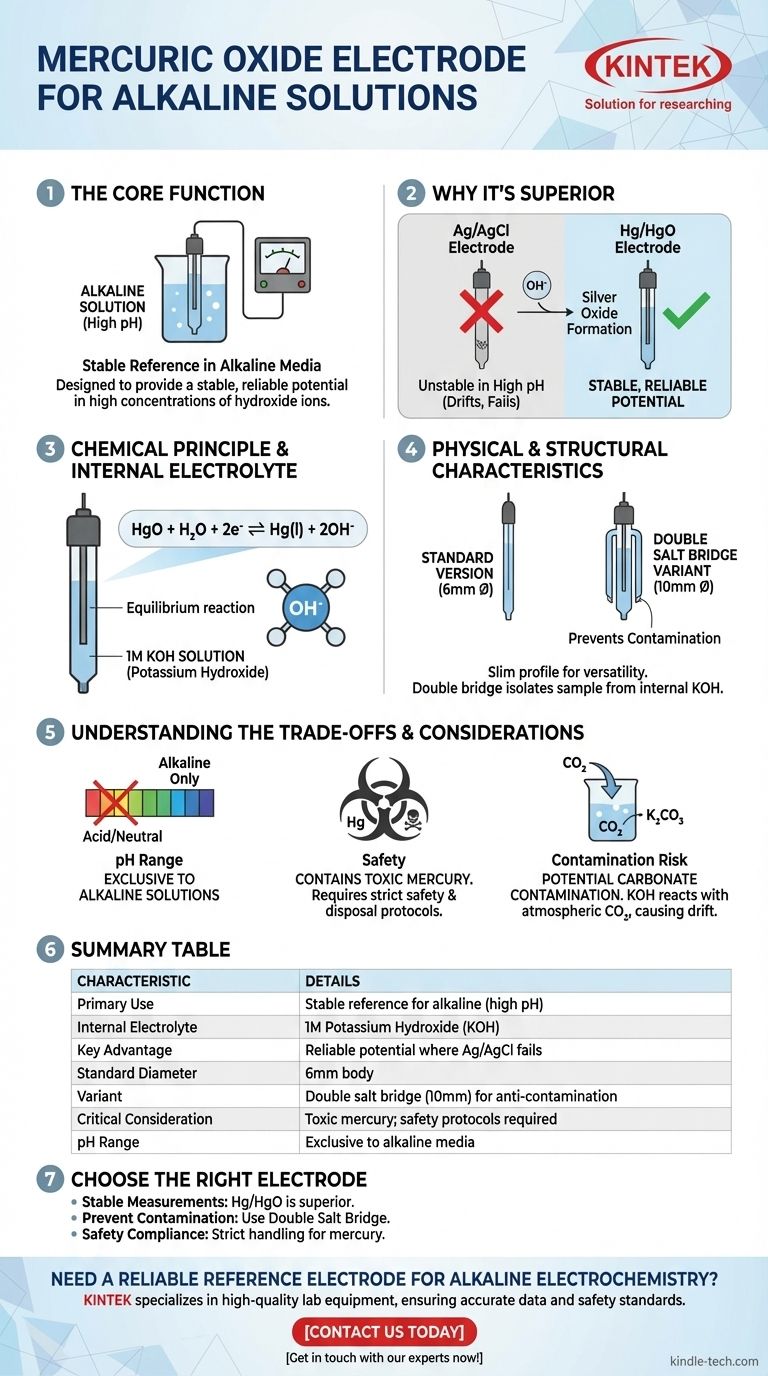
The primary characteristic of a mercuric oxide (Hg/HgO) electrode is its specific design as a stable reference electrode for use in alkaline solutions. It is typically filled with a 1M potassium hydroxide (KOH) solution and is built to avoid the chemical instability that affects other common reference electrodes in high pH environments.
The mercuric oxide electrode is the standard choice for alkaline electrochemistry because it provides a stable, reliable potential in high concentrations of hydroxide ions, a condition where more common electrodes like silver/silver chloride (Ag/AgCl) fail.

The Core Function: A Stable Reference in Alkaline Media
To understand the Hg/HgO electrode, one must first understand the problem it solves. Standard reference electrodes often perform poorly in strongly basic (alkaline) solutions.
Why Standard Electrodes Can Fail
Common reference electrodes, such as the Ag/AgCl or Saturated Calomel Electrode (SCE), contain chloride ions. In highly alkaline solutions, hydroxide ions (OH⁻) can react with the internal components of these electrodes (e.g., forming silver oxide), causing the reference potential to drift and become unreliable.
The Chemical Principle of Hg/HgO
The mercuric oxide electrode establishes a stable potential based on the following equilibrium reaction: HgO + H₂O + 2e⁻ ⇌ Hg(l) + 2OH⁻
This reaction directly involves the hydroxide ions of the alkaline solution, making its potential inherently stable and well-defined within that specific chemical environment.
The Internal Filling Solution
The defining internal electrolyte for this electrode is a potassium hydroxide (KOH) solution, typically at a concentration of 1 mole per liter (1M/L). This ensures a constant and known hydroxide ion activity inside the electrode.
Physical and Structural Characteristics
The physical design of the electrode is straightforward but includes important variations for specific applications.
Standard Dimensions
The standard version of the mercuric oxide electrode typically features a body with a 6mm diameter. This slim profile is suitable for a wide range of laboratory cells and setups.
The Double Salt Bridge Variant
A double salt bridge version, often with a larger 10mm diameter, is also available. This design is crucial when the internal 1M KOH filling solution could contaminate the sample being measured, or when ions from the sample could contaminate the electrode. The outer bridge can be filled with an inert electrolyte compatible with the sample.
Understanding the Trade-offs
While ideal for alkaline solutions, the Hg/HgO electrode is not a universal solution and comes with critical considerations.
Limited pH Range
This electrode is a specialist. It is designed exclusively for alkaline solutions and should not be used in acidic or neutral media, where its potential is not stable and it can be damaged. For acidic conditions, an electrode like the mercurous sulfate electrode is used instead.
Environmental and Safety Concerns
The most significant drawback is its composition. The electrode contains mercury and mercuric oxide, which are highly toxic. Strict safety protocols for handling and, especially, disposal are mandatory to prevent environmental contamination and health risks.
Potential for Carbonate Contamination
The KOH electrolyte can react with carbon dioxide (CO₂) from the atmosphere to form potassium carbonate. Over time, this contamination can slowly alter the concentration of hydroxide ions and cause the reference potential to drift.
Making the Right Choice for Your Alkaline System
Your choice of reference electrode must be deliberate and aligned with your experimental requirements.
- If your primary focus is stable measurements in high pH solutions: The mercuric oxide electrode is the technically superior and most reliable choice over Ag/AgCl or SCE.
- If your primary focus is preventing cross-contamination: Select the double salt bridge version to isolate your sample from the electrode's internal KOH filling solution.
- If your primary focus is safety and environmental compliance: You must implement proper handling and disposal procedures due to the electrode's mercury content.
Ultimately, using a mercuric oxide electrode demonstrates a careful and informed approach to achieving accurate electrochemical data in alkaline environments.
Summary Table:
| Characteristic | Details |
|---|---|
| Primary Use | Stable reference electrode for alkaline solutions (high pH) |
| Internal Electrolyte | 1M Potassium Hydroxide (KOH) solution |
| Key Advantage | Stable, reliable potential where Ag/AgCl electrodes fail |
| Standard Diameter | 6mm body |
| Variant | Double salt bridge version (10mm) to prevent contamination |
| Critical Consideration | Contains toxic mercury; requires strict safety protocols |
| pH Range | Exclusive to alkaline media; not for acidic/neutral solutions |
Need a reliable reference electrode for your alkaline electrochemistry work?
KINTEK specializes in high-quality lab equipment, including mercuric oxide electrodes designed for stable performance in high pH environments. Our products ensure accurate data for your research while adhering to strict safety standards.
Contact us today to find the right electrode for your laboratory needs and enhance the precision of your alkaline system measurements.
Get in touch with our experts now!
Visual Guide
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Gold Disc Electrode
People Also Ask
- Which electrode is used as a reference? A Guide to Accurate Electrochemical Measurements
- What is the reference electrode for mercury mercurous sulfate? A Guide to Chloride-Free Electrochemistry
- What are the general precautions for using a reference electrode? Ensure Stable Potentials for Accurate Data
- Which type of electrode can be used as a reference point? Select the Right One for Accurate Measurements
- Why and how should the electrodes of an electrolytic cell be calibrated? Ensure Reliable Results



















