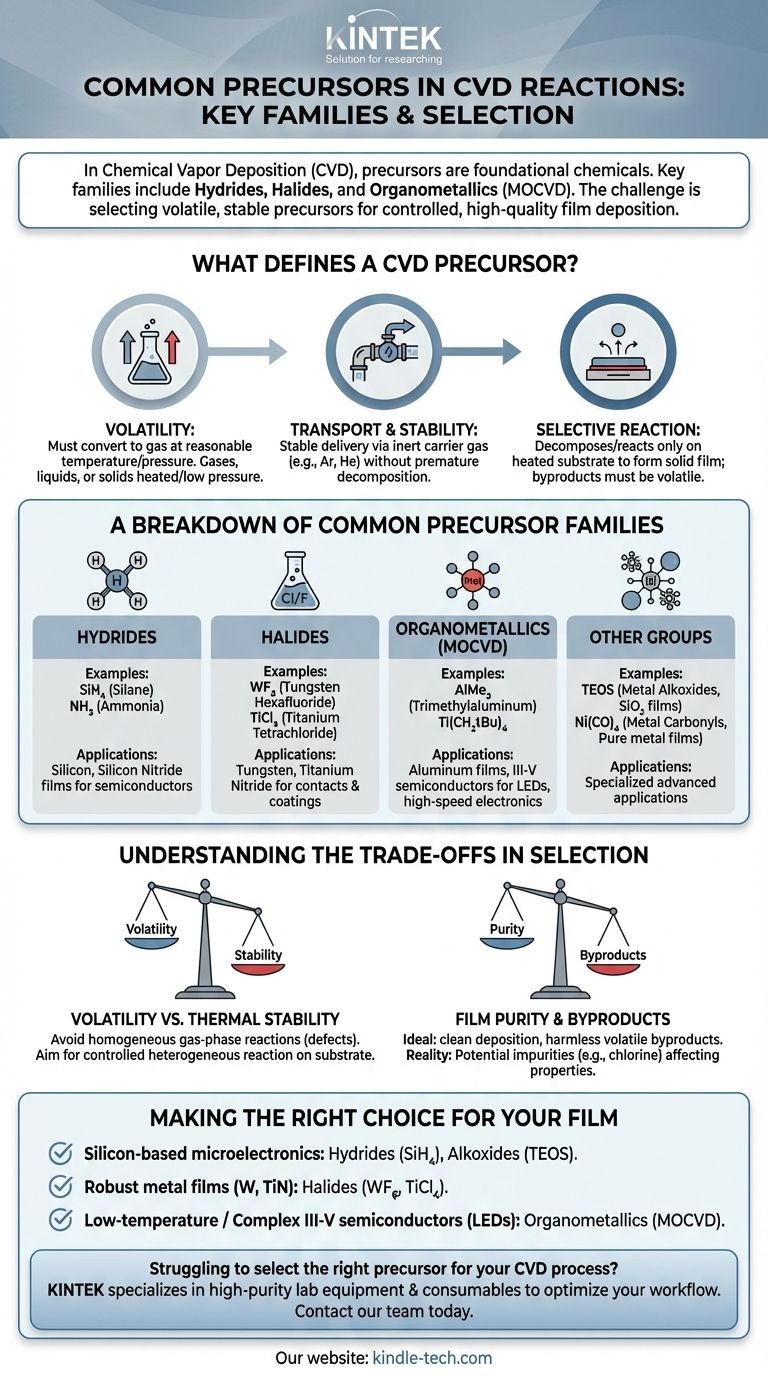
In Chemical Vapor Deposition (CVD), the most common precursors are grouped into several key chemical families. These include simple hydrides like silane (SiH₄), halides like tungsten hexafluoride (WF₆), and organometallics such as trimethylaluminum (AlMe₃), which are central to Metal-Organic CVD (MOCVD). Other important classes are metal alkoxides, metal carbonyls, and metal dialkylamides.
The core challenge of CVD is not just finding a chemical that contains the element you want to deposit. The true task is selecting a precursor that is volatile enough to be transported as a gas but stable enough not to react until it reaches the heated substrate, ensuring a controlled, high-quality film deposition.

What Defines a CVD Precursor?
A precursor is the foundational chemical that carries the desired element to a substrate. To be effective, it must possess a specific set of physical and chemical properties.
The Essential Property: Volatility
A precursor must be volatile, meaning it can be converted into a gaseous or vapor state at a reasonable temperature and pressure. This is non-negotiable, as CVD is, by definition, a vapor-phase process.
Precursors can start as a gas, liquid, or solid. Gases are the simplest to use, while liquids and solids require heating or low-pressure environments to generate sufficient vapor for transport to the reaction chamber.
Transport and Stability
Once in a gaseous state, the precursor must be stable enough to be delivered to the reactor without decomposing prematurely.
Often, an inert carrier gas like argon (Ar) or helium (He) is used. This gas helps transport the precursor vapor and can prevent unwanted side-reactions, like oxidation, before the precursor reaches the intended surface.
Selective Reaction on the Substrate
The ideal precursor decomposes or reacts only on the heated substrate (heterogeneous reaction) to form a solid film. The other elements within the precursor molecule should form volatile byproducts that are easily removed from the chamber.
A Breakdown of Common Precursor Families
The choice of precursor family is dictated by the material to be deposited, the required deposition temperature, and the desired film purity.
Hydrides (e.g., SiH₄, GeH₄, NH₃)
Hydrides are compounds containing hydrogen bonded to another element. They are foundational for the semiconductor industry.
Silane (SiH₄) is the workhorse precursor for depositing silicon (Si) and silicon dioxide (SiO₂) films. Ammonia (NH₃) is commonly used as a nitrogen source for silicon nitride (Si₃N₄) films.
Halides (e.g., WF₆, TiCl₄, H₂SiCl₂)
Halides are compounds containing a halogen (F, Cl, Br, I). They are widely used for depositing metals and silicon-based materials.
Tungsten hexafluoride (WF₆) is the standard for depositing tungsten films, which are used for electrical contacts. Titanium tetrachloride (TiCl₄) is used for titanium nitride (TiN), a hard coating and diffusion barrier.
Organometallics (e.g., AlMe₃, Ti(CH₂tBu)₄)
Organometallics contain a metal-carbon bond and are the defining feature of Metal-Organic CVD (MOCVD). This technique often allows for lower deposition temperatures than traditional CVD.
They are critical for depositing complex compound semiconductors, like those used in LEDs and high-speed electronics. Trimethylaluminum (AlMe₃) is a classic example used for depositing aluminum-containing films.
Other Important Precursor Groups
Several other families serve specialized purposes.
Metal alkoxides like TEOS (tetraethyl orthosilicate) are liquid sources used for high-quality silicon dioxide films. Metal carbonyls like nickel carbonyl (Ni(CO)₄) are used for depositing pure metal films. Metal dialkylamides and metal diketonates are also used in specific advanced applications.
Understanding the Trade-offs in Precursor Selection
Choosing a precursor involves balancing competing factors. An error in selection can lead to poor film quality, contamination, or process failure.
Volatility vs. Thermal Stability
This is the primary trade-off. A precursor must be volatile enough to transport but not so unstable that it decomposes in the gas lines before reaching the substrate. This premature decomposition is known as a homogeneous reaction.
Homogeneous reactions are highly undesirable as they create particles in the gas phase, which can fall onto the substrate and create defects in the film. The goal is always a controlled heterogeneous reaction on the substrate surface.
Film Purity and Byproducts
An ideal precursor deposits the target element cleanly, while all other components form harmless, volatile byproducts.
In reality, byproducts can sometimes react with the film or incorporate into it as impurities. For example, using chlorine-based precursors (halides) can sometimes lead to chlorine contamination in the final film, affecting its electrical properties.
Substrate and Temperature Compatibility
The precursor’s decomposition temperature must be compatible with the substrate's thermal stability.
You cannot use a high-temperature precursor to coat a temperature-sensitive substrate, like a polymer, as the substrate would be damaged or destroyed. This is a key reason for the development of low-temperature MOCVD and Plasma-Enhanced CVD (PECVD) processes.
Making the Right Choice for Your Film
Your choice of precursor is fundamentally tied to the material you intend to grow and the process conditions you can tolerate.
- If your primary focus is standard silicon-based microelectronics: You will almost certainly rely on hydrides like silane (SiH₄) and alkoxides like TEOS.
- If your primary focus is depositing robust metal films like tungsten or titanium nitride: Halides such as WF₆ and TiCl₄ are the industry-standard choice.
- If your primary focus is low-temperature deposition or complex III-V semiconductors (for LEDs/lasers): You will need to use organometallic precursors in an MOCVD process.
Ultimately, selecting the correct precursor is the first and most critical step in controlling the final properties and quality of your deposited material.
Summary Table:
| Precursor Family | Key Examples | Common Applications |
|---|---|---|
| Hydrides | SiH₄, NH₃ | Silicon, silicon nitride films for semiconductors |
| Halides | WF₆, TiCl₄ | Tungsten, titanium nitride for contacts & coatings |
| Organometallics | AlMe₃ | Aluminum films, III-V semiconductors for LEDs |
| Metal Alkoxides | TEOS | High-quality silicon dioxide films |
| Metal Carbonyls | Ni(CO)₄ | Pure metal film deposition |
Struggling to select the right precursor for your CVD process? KINTEK specializes in providing high-purity lab equipment and consumables tailored to your laboratory's unique deposition needs. Whether you're working with hydrides for semiconductors or organometallics for advanced materials, our expertise ensures you achieve controlled, high-quality film deposition. Contact our team today to discuss how our solutions can optimize your CVD workflow and enhance your research outcomes.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
People Also Ask
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- What is the difference between plasma CVD and thermal CVD? Choose the Right Method for Your Substrate
- How do PECVD systems improve DLC coatings on implants? Superior Durability and Biocompatibility Explained
- What is the difference between PECVD and APCVD? Choose the Right CVD Method for Your Application
- What are different types of thin films? A Guide to Function, Material, and Deposition Methods



















