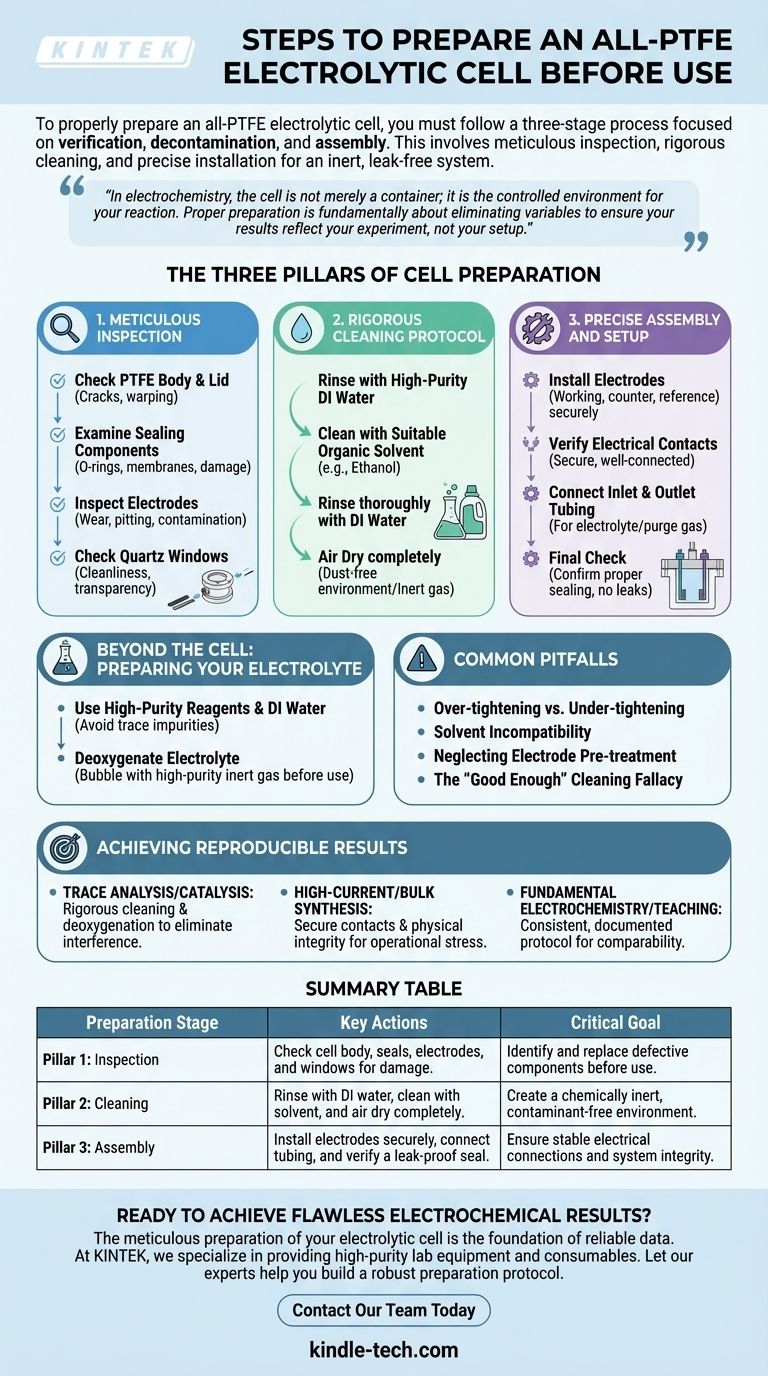
To properly prepare an all-PTFE electrolytic cell, you must follow a three-stage process focused on verification, decontamination, and assembly. This involves a meticulous inspection of all components for damage, a rigorous cleaning protocol using appropriate solvents and deionized water, and a precise installation of the electrodes and seals to ensure an inert, leak-free system.
In electrochemistry, the cell is not merely a container; it is the controlled environment for your reaction. Proper preparation is fundamentally about eliminating variables—contamination, leaks, and electrical instability—to ensure your results reflect your experiment, not your setup.

The Three Pillars of Cell Preparation
A successful experiment begins long before the first volt is applied. The integrity of your data relies on a systematic and consistent preparation protocol built on three core principles.
Pillar 1: Meticulous Inspection
Before any cleaning or assembly, conduct a full inventory and inspection of every component. A minor defect found now can save an entire experiment later.
Check the PTFE cell body and lid for any cracks, warping, or deep scratches. The integrity of the main vessel is paramount.
Examine all sealing components, such as O-rings or ion-exchange membranes. These are often the first points of failure. If they appear compressed, brittle, or damaged in any way, replace them immediately.
Inspect the electrodes for physical wear, pitting, or surface contamination. An oxidized or contaminated electrode surface will produce unreliable data.
If your cell includes quartz windows, ensure they are clean, transparent, and free of cracks to allow for accurate spectroscopic measurements.
Pillar 2: Rigorous Cleaning Protocol
The goal of cleaning is to create a chemically inert environment. Contaminants from previous experiments, manufacturing, or handling can introduce unwanted side reactions.
Begin by rinsing the cell components with high-purity deionized (DI) water to remove soluble salts and particulates.
Next, clean the cell with a suitable organic solvent, such as ethanol or isopropanol, to remove grease and organic residues. Soaking may be necessary for stubborn contamination.
Follow the solvent wash with another thorough rinse using DI water.
Finally, allow the components to air dry completely in a dust-free environment. For faster or more stringent applications, you can gently dry them with a stream of high-purity nitrogen or argon gas.
Pillar 3: Precise Assembly and Setup
Correct assembly ensures a leak-proof seal and stable electrical connections, which are critical for accurate measurements.
Carefully install the working, counter, and reference electrodes into their designated ports. Ensure they are positioned correctly as specified by your experimental design.
Verify that all electrical contacts are secure and well-connected. A loose connection introduces resistance and signal noise, compromising your data.
Connect any inlet and outlet tubing for electrolyte or purge gas. Once assembled, perform a final check to confirm the cell is properly sealed and free from leaks.
Beyond the Cell: Preparing Your Electrolyte
An impeccably clean cell is easily compromised by an impure electrolyte. The electrolyte must be prepared with the same level of care.
The Importance of Purity
Always formulate your electrolyte using high-purity chemical reagents and deionized or distilled water. Trace impurities in low-grade reagents can act as catalysts, inhibitors, or redox interferents.
Pre-Treatment and Filling
For many experiments, dissolved oxygen is a significant interferent. Deoxygenate the electrolyte by bubbling a high-purity inert gas (like nitrogen or argon) through it before adding it to the cell.
When ready, carefully pour the electrolyte into the cell. Avoid splashing onto the electrode contacts and do not overfill past the recommended volume.
Understanding the Common Pitfalls
Even experienced researchers can make mistakes during setup. Being aware of these common pitfalls helps prevent them.
Over-tightening vs. Under-tightening
When assembling the cell, it's tempting to tighten components excessively to prevent leaks. However, this can deform the PTFE body, damage the threads, or crush delicate seals. Conversely, an under-tightened cell will leak. The goal is a firm, "hand-tight" seal.
Solvent Incompatibility
While PTFE is highly inert, other components like sealing rings or membranes may not be. Always verify that your chosen cleaning solvent is compatible with all wetted parts of your cell to prevent degradation.
Neglecting Electrode Pre-treatment
The electrodes themselves often require a separate preparation step, such as polishing, acid washing, or electrochemical cycling, to achieve a clean and active surface. Simply placing them in a clean cell is often not enough.
The "Good Enough" Cleaning Fallacy
In sensitive experiments like trace analysis or catalysis, even minute levels of contamination can skew results. A quick rinse is rarely sufficient. A consistent, documented cleaning protocol is your best defense against irreproducible data.
Achieving Reproducible Results
Your preparation strategy should align directly with your experimental goals to ensure the integrity of your findings.
- If your primary focus is trace analysis or catalysis: Your highest priority is a rigorous cleaning protocol and electrolyte deoxygenation to eliminate any possible source of chemical interference.
- If your primary focus is high-current electrolysis or bulk synthesis: Your highest priority is verifying secure electrode contacts and the physical integrity of seals and membranes to handle operational stress without failing.
- If your primary focus is fundamental electrochemistry or teaching: Your highest priority is developing a consistent, documented preparation protocol that ensures results are comparable and reproducible across multiple experiments.
A well-prepared cell is the foundation upon which reliable and meaningful electrochemical data is built.
Summary Table:
| Preparation Stage | Key Actions | Critical Goal |
|---|---|---|
| Pillar 1: Inspection | Check cell body, seals, electrodes, and windows for damage. | Identify and replace defective components before use. |
| Pillar 2: Cleaning | Rinse with DI water, clean with solvent (e.g., ethanol), and air dry completely. | Create a chemically inert, contaminant-free environment. |
| Pillar 3: Assembly | Install electrodes securely, connect tubing, and verify a leak-proof seal. | Ensure stable electrical connections and system integrity. |
Ready to achieve flawless electrochemical results?
The meticulous preparation of your electrolytic cell is the foundation of reliable data. At KINTEK, we specialize in providing the high-purity lab equipment and consumables—from PTFE cells and electrodes to high-purity solvents and reagents—that your research demands.
Let our experts help you build a robust preparation protocol. Contact our team today to discuss your specific laboratory needs and ensure your experiments are set up for success from the very first step.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Custom PTFE Teflon Parts Manufacturer for Non-Standard Insulator Customization
- Platinum Auxiliary Electrode for Laboratory Use
- Li-Air Battery Case for Battery Lab Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
People Also Ask
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- What is the purpose of using a frit glass tube in a three-electrode cell? Enhance Vanadium Redox Testing Accuracy
- What is the precaution regarding temperature when using an all-PTFE electrolytic cell? Essential Thermal Safety Tips
- Which parameters must be strictly controlled using an all-PTFE electrolytic cell? Ensure Precision and Safety
- What role does a two-electrode electrochemical reactor play in TiO2 growth? Achieve Ordered Nanostructures Today



















