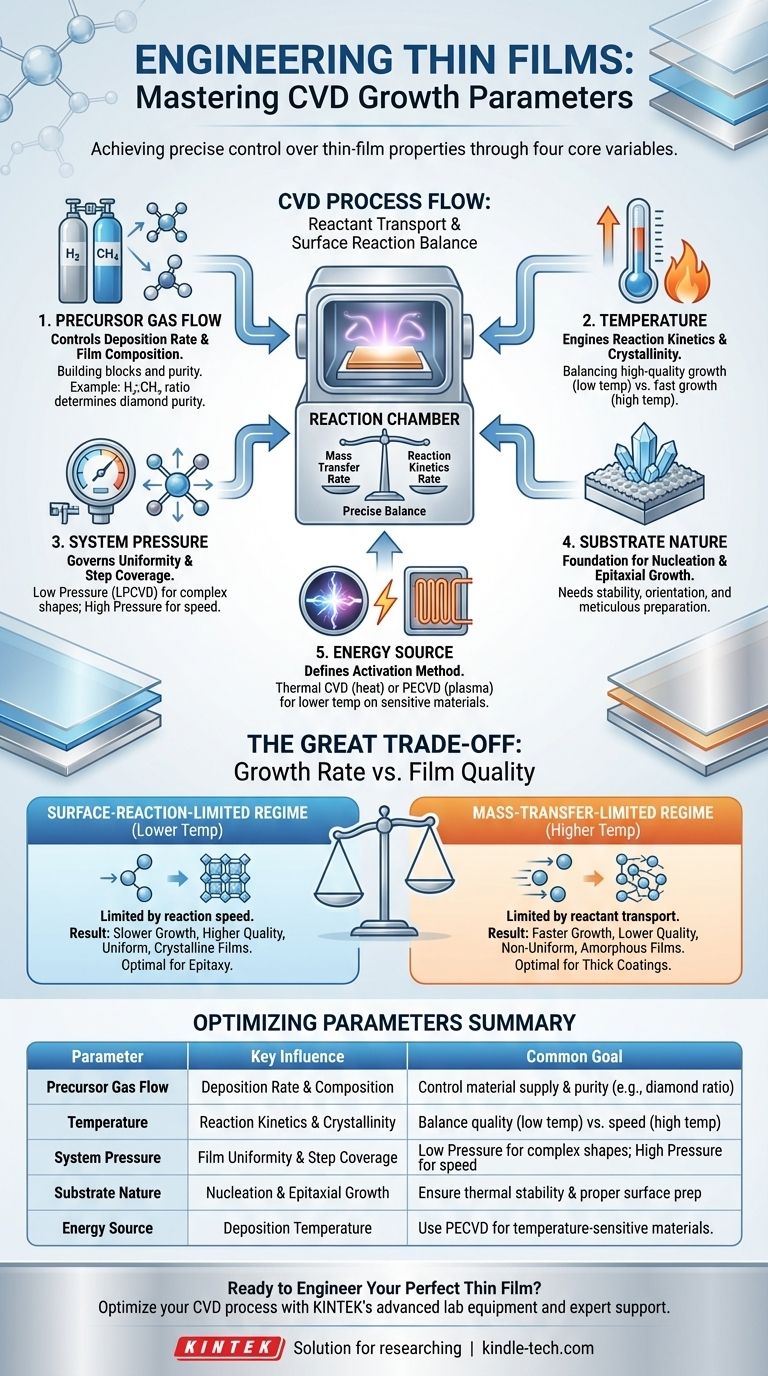
In short, the primary controllable parameters for Chemical Vapor Deposition (CVD) are the flow rates of precursor gases, the system temperature, the chamber pressure, and the nature of the substrate. These variables are adjusted to control the chemical reactions that form a solid film on a surface, directly influencing the final material's thickness, quality, and properties.
The goal of controlling CVD parameters is to strike a precise balance between two competing phenomena: the rate at which reactant gases are transported to the surface (mass transfer) and the rate at which they react on that surface (reaction kinetics). Mastering this balance is the key to engineering a film with your desired characteristics.

The Core Parameters of CVD Control
Understanding how each parameter influences the deposition process allows you to move from simply performing CVD to truly engineering a material. The process generally involves introducing reactants, activating them, allowing them to react on a substrate, and removing byproducts. Each parameter is a lever that adjusts one or more of these stages.
Precursor Gases: The Building Blocks
The precursor gases are the chemical building blocks of your final film. They consist of one or more reactants and often a carrier gas.
The composition of the gas determines what material you can grow. For example, growing silicon nitride (Si₃N₄) might use silane (SiH₄) and ammonia (NH₃) as precursors.
The gas flow rate dictates the concentration of reactants in the chamber. A higher flow rate increases the supply of material to the substrate, which can increase the deposition rate.
Finally, the ratio between different precursor gases is critical. In diamond CVD, a hydrogen-to-methane ratio of 99:1 is common. The excess hydrogen selectively etches away lower-quality, non-diamond carbon, dramatically improving the purity of the final diamond film.
Temperature: The Engine of Reaction
Temperature is arguably the most critical parameter in thermal CVD, as it provides the activation energy needed to initiate chemical reactions.
Substrate temperature directly controls the rate of the surface reaction. At lower temperatures, reactions are slow, which is known as the reaction-limited regime. This often produces very uniform, high-quality films. As temperature increases, the reaction rate speeds up dramatically.
Chamber temperature can also be a factor. If the gas in the chamber gets too hot before reaching the substrate, unwanted reactions can occur in the gas phase, creating particles that can fall onto and contaminate your film.
System Pressure: Controlling the Environment
The pressure inside the reaction chamber governs the behavior and transport of the gas molecules.
Lowering the pressure increases the mean free path of gas molecules—the average distance they travel before colliding with another molecule. In Low-Pressure CVD (LPCVD), this longer path allows reactants to travel further and coat complex, three-dimensional surfaces more uniformly.
Conversely, higher pressure (like in Atmospheric Pressure CVD, or APCVD) leads to a shorter mean free path and a higher concentration of reactants near the substrate. This can enable very fast growth rates but often with poorer uniformity on complex shapes.
The Substrate: The Foundation for Growth
The substrate is not a passive observer; it is an active participant in the CVD process.
The material choice is foundational. The substrate must be able to withstand the process temperatures and may even act as a catalyst for the desired reaction.
Its crystallographic orientation can serve as a template. In a process called epitaxy, the deposited film can adopt the same crystal structure as the substrate, leading to a highly ordered, single-crystal layer.
Surface preparation is non-negotiable for high-quality growth. The surface must be meticulously cleaned to remove contaminants. Sometimes, it is intentionally "seeded" (e.g., by polishing with diamond powder) to create nucleation sites that encourage film growth.
Energy Source: The Activation Method
To react, precursor gases must be broken down into more reactive species, or radicals. The method of activation defines the type of CVD process.
In Thermal CVD, high temperature is the sole energy source. In Plasma-Enhanced CVD (PECVD), an electrical field creates a plasma that breaks down gases. This allows for deposition at much lower temperatures, making it suitable for temperature-sensitive substrates like polymers. Other methods include using a hot filament or a laser to provide the activation energy.
Understanding the Trade-offs: Growth Rate vs. Film Quality
The central challenge in optimizing CVD is managing the trade-off between deposition speed and film quality. The process operates in one of two main regimes, which are controlled by temperature.
The Surface-Reaction-Limited Regime
At lower temperatures, the deposition rate is limited by how fast the chemical reaction can occur on the substrate surface. There are plenty of reactants available, but they lack the thermal energy to react quickly.
- Result: Slower growth, but typically higher quality, more uniform, and more crystalline films.
The Mass-Transfer-Limited Regime
At higher temperatures, the surface reaction becomes extremely fast. The bottleneck is no longer the reaction itself but the rate at which fresh reactant molecules can be transported through the gas to the substrate surface.
- Result: Very fast growth, but the film quality can suffer. The rapid, diffusion-controlled growth can lead to lower density, poorer uniformity, and amorphous (non-crystalline) structures.
Optimizing Parameters for Your Goal
Your choice of parameters should be dictated entirely by the desired properties of your final film.
- If your primary focus is the highest possible film quality (e.g., for semiconductor epitaxy): Operate in the surface-reaction-limited regime with lower temperatures and controlled, lower precursor flow rates to ensure ordered, uniform growth.
- If your primary focus is the fastest possible deposition rate (e.g., for thick, protective coatings): Operate in the mass-transfer-limited regime using higher temperatures and high gas flow rates, accepting a potential trade-off in structural perfection.
- If your primary focus is depositing on a temperature-sensitive substrate (e.g., a plastic or polymer): Use a non-thermal activation method like PECVD, which allows for deposition at significantly lower substrate temperatures.
By understanding these parameters as levers to control the underlying physics and chemistry, you can effectively engineer materials to meet your specific needs.
Summary Table:
| Parameter | Key Influence | Common Goal |
|---|---|---|
| Precursor Gas Flow | Deposition Rate & Film Composition | Control material supply and purity (e.g., H₂:CH₄ ratio for diamond). |
| Temperature | Reaction Kinetics & Crystallinity | Balance high-quality growth (low temp) vs. fast growth (high temp). |
| System Pressure | Film Uniformity & Step Coverage | Low Pressure (LPCVD) for complex shapes; High Pressure for speed. |
| Substrate Nature | Nucleation & Epitaxial Growth | Ensure thermal stability and proper surface preparation. |
| Energy Source | Deposition Temperature | Use Plasma-Enhanced CVD (PECVD) for temperature-sensitive materials. |
Ready to Engineer Your Perfect Thin Film?
Optimizing CVD parameters is the key to achieving your specific material goals, whether you prioritize ultimate film quality, maximum deposition speed, or compatibility with sensitive substrates.
KINTEK specializes in providing the advanced lab equipment and expert support you need to master your CVD processes. We help our customers in research and industry unlock precise control over their thin-film deposition.
Let's discuss your application. Contact our experts today to explore how our solutions can help you achieve superior and reproducible results.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Laboratory CVD Boron Doped Diamond Materials
People Also Ask
- Why does a PECVD vacuum system require both a rotary vane and turbo pump? Ensure High-Purity Coatings
- Why is a Matching Network Indispensable in RF-PECVD for Siloxane Films? Ensure Stable Plasma and Uniform Deposition
- Can plasma enhanced CVD deposit metals? Why PECVD is rarely used for metal deposition
- What are different types of thin films? A Guide to Function, Material, and Deposition Methods
- What are the process capabilities of ICPCVD systems? Achieve Low-Damage Film Deposition at Ultra-Low Temperatures



















