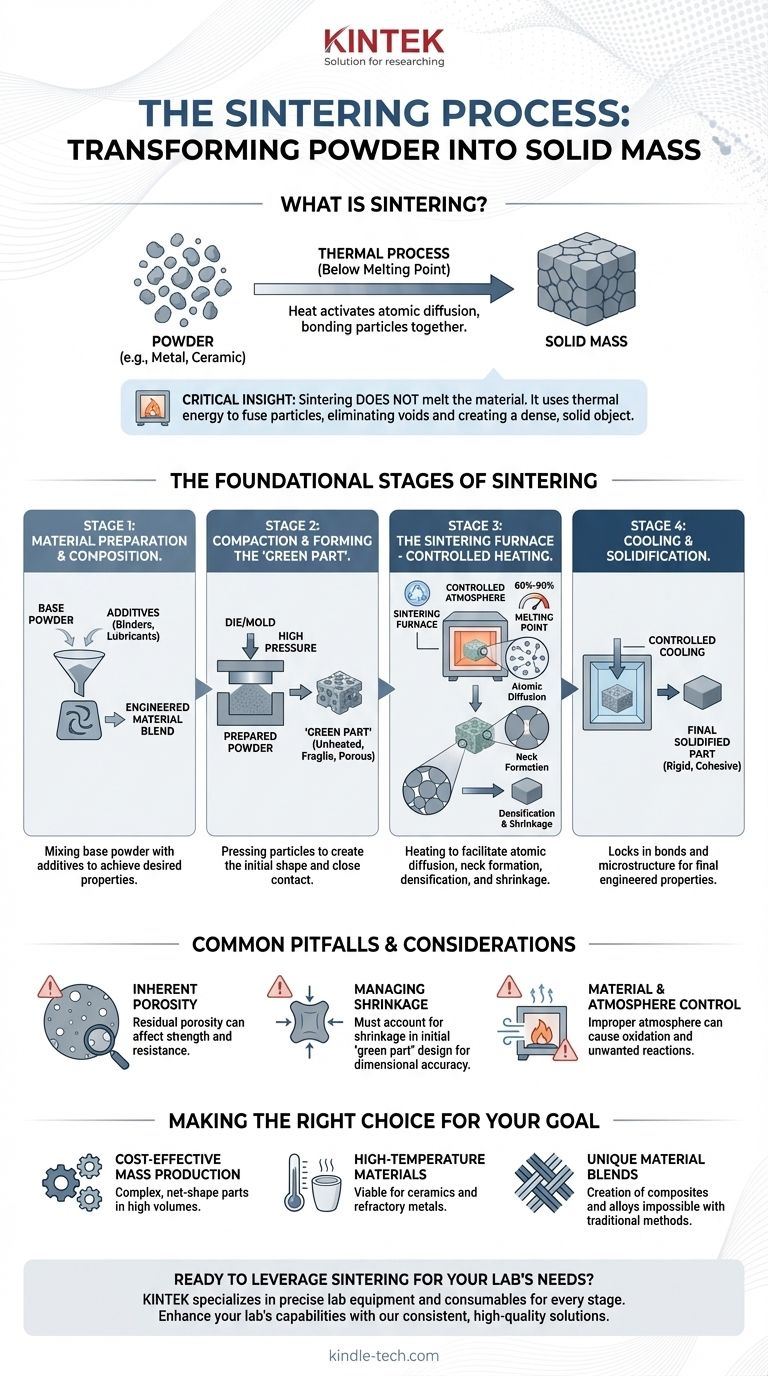
In essence, sintering is a thermal process that transforms a powder into a solid mass. It achieves this by heating the material to a temperature below its melting point, which causes the individual particles to bond together. The fundamental process can be broken down into three core stages: preparing and forming the powder, heating it in a controlled environment, and finally, cooling the solidified part.
The critical insight is that sintering does not melt the material. Instead, it uses thermal energy to activate atomic diffusion, causing individual particles to fuse, eliminating the voids between them and creating a dense, solid object.

The Foundational Stages of Sintering
To understand the process, it's best to think of it as a journey from a loose collection of particles to a single, cohesive component. Each stage plays a critical role in determining the final properties of the part.
Stage 1: Material Preparation and Composition
Before any forming can occur, the raw material must be prepared. This involves selecting the base powder and often mixing it with other elements or additives.
These additives can include binders to provide initial strength or lubricants to assist in the compaction stage. The precise composition is engineered to achieve the desired final mechanical and physical properties.
Stage 2: Compaction and Forming the "Green Part"
The prepared powder is then loaded into a die or mold and compacted under high pressure. The goal is to press the particles into close contact, creating the initial shape of the component.
This unheated, fragile, and highly porous piece is known as the "green part." While it holds its shape, it has very little mechanical strength and is merely a precursor to the final product.
Stage 3: The Sintering Furnace - Controlled Heating
This is the heart of the process. The green part is placed in a furnace with a controlled atmosphere and heated to a specific temperature, typically between 60% and 90% of the material's melting point.
At this temperature, atoms begin to migrate across the boundaries of the contacting particles. This process, called atomic diffusion, forms "necks" at the particle contact points, which gradually grow until the individual particles merge.
As the particles fuse, the voids (or pores) between them shrink or close up entirely. This leads to densification, where the part becomes stronger and more solid, and shrinkage, where its overall volume decreases.
Stage 4: Cooling and Solidification
After being held at the sintering temperature for a predetermined time, the component is cooled in a controlled manner.
This final stage locks the newly formed bonds and microstructure in place, allowing the part to solidify into a rigid and cohesive structure with its final engineered properties.
Common Pitfalls and Considerations
While powerful, the sintering process has inherent characteristics that must be managed to ensure a successful outcome. Understanding these trade-offs is crucial for any engineering application.
Inherent Porosity
Complete densification is not always achieved. Some residual porosity (tiny voids) can remain in the final part, which can act as stress concentration points and may affect properties like tensile strength and fatigue resistance.
Managing Shrinkage
Because the part shrinks as it densifies, the initial "green part" must be designed slightly larger than the desired final dimensions. Accurately predicting and controlling this shrinkage is critical for achieving tight dimensional tolerances.
Material and Atmosphere Control
The success of sintering depends heavily on the material being processed and the atmosphere within the furnace. An improper atmosphere can lead to oxidation or other unwanted chemical reactions that compromise the integrity of the final part.
Making the Right Choice for Your Goal
Sintering is not a one-size-fits-all solution. Its advantages are most pronounced when applied to specific challenges in manufacturing and material science.
- If your primary focus is cost-effective mass production: Sintering is exceptional for creating complex, net-shape metal parts in high volumes with minimal material waste and reduced need for secondary machining.
- If your primary focus is high-temperature materials: It is one of the few viable methods for shaping ceramics and refractory metals that have melting points too high for practical casting.
- If your primary focus is creating unique material blends: The process allows for the creation of metal matrix composites and alloys that would be impossible to produce through traditional melting and casting.
By understanding these core principles, you can effectively leverage sintering to transform powdered materials into robust, high-performance components.
Summary Table:
| Stage | Key Action | Outcome |
|---|---|---|
| 1. Preparation | Mixing base powder with additives | Engineered material blend |
| 2. Compaction | Pressing powder in a die under high pressure | Formation of the fragile "green part" |
| 3. Sintering | Heating in a controlled atmosphere below melting point | Particle fusion, densification, and shrinkage |
| 4. Cooling | Controlled solidification in the furnace | Final part with locked-in properties |
Ready to leverage sintering for your lab's material production needs?
KINTEK specializes in providing the precise lab equipment and consumables required for every stage of the sintering process. Whether you are developing new material blends, producing complex ceramic components, or need reliable furnace atmospheres, our expertise ensures you achieve consistent, high-quality results.
Contact our experts today to discuss how we can support your sintering projects and enhance your lab's capabilities.
Visual Guide
Related Products
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
People Also Ask
- What are the advantages of vacuum sintering? Achieve Superior Purity, Strength, and Performance
- How does precise temperature control affect FeCoCrNiMnTiC high-entropy alloys? Master Microstructural Evolution
- What is the impact factor of powder metallurgy progress? A 2022 Analysis & Context
- How does a vacuum hot press sintering furnace facilitate a high-quality bond? Achieve Superior Metallurgical Coating
- How does a vacuum environment system contribute to the hot pressing sintering of B4C-CeB6? Unlock Peak Ceramic Density



















