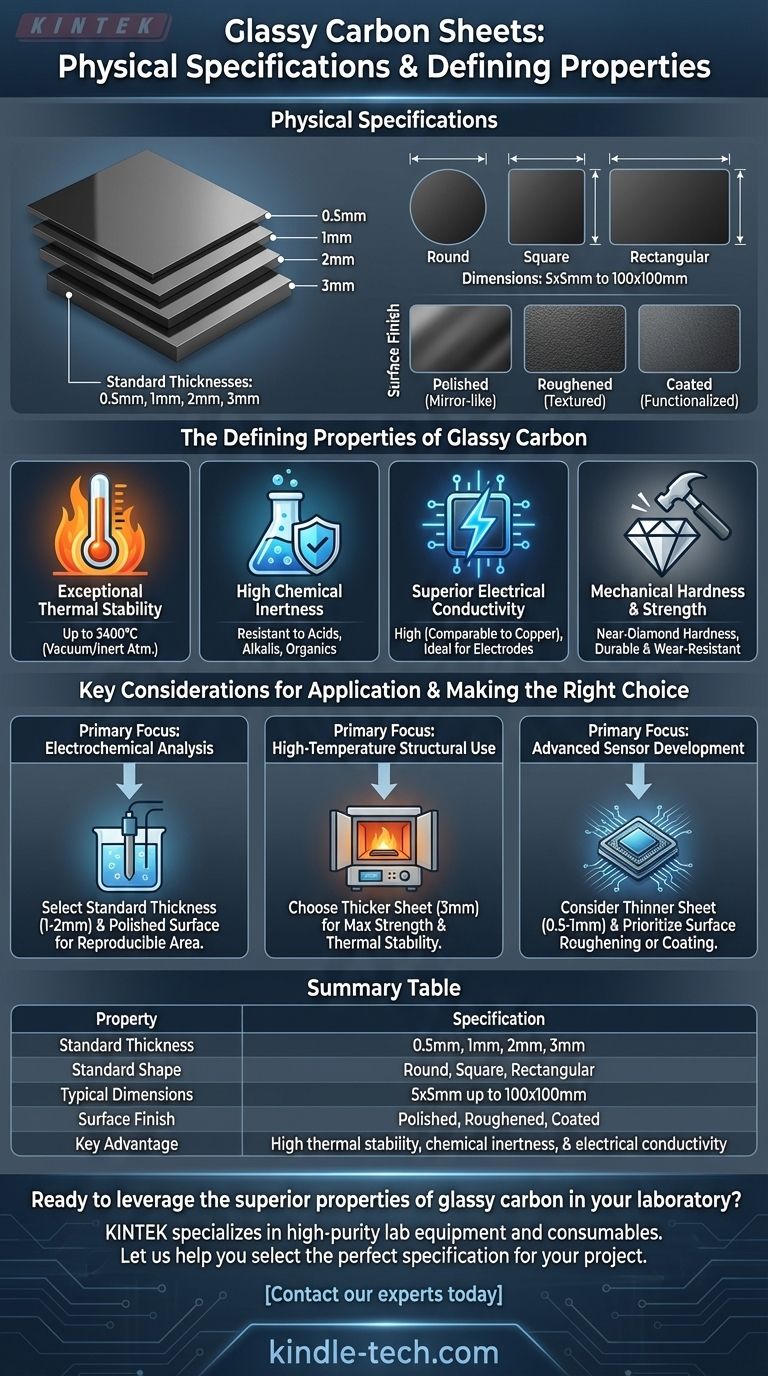
In terms of physical specifications, glassy carbon sheets are typically available in standard thicknesses of 0.5mm, 1mm, 2mm, and 3mm. These sheets come in round, square, or rectangular forms with dimensions ranging from 5x5mm up to 100x100mm, and their surfaces can be polished, roughened, or coated to suit specific experimental needs.
While the physical dimensions are standardized, the true value of glassy carbon lies in its unique combination of properties. Understanding its thermal, chemical, and electrical characteristics is the key to leveraging it correctly in demanding scientific and industrial applications.

The Defining Properties of Glassy Carbon
Glassy carbon, also known as vitreous carbon, is a non-graphitizing, amorphous form of carbon. Its unique atomic structure gives it a powerful and distinct set of characteristics that separate it from other carbon forms like graphite or diamond.
Exceptional Thermal Stability
Glassy carbon demonstrates incredible resistance to high temperatures. It can withstand up to 3400°C in a vacuum or inert atmosphere, making it a superior choice for high-temperature crucibles, furnace components, and structural supports.
High Chemical Inertness
This material exhibits outstanding oxidation resistance and chemical stability. It is inert in a wide range of acidic, alkaline, and organic environments, ensuring reactions are not contaminated by the material itself.
Superior Electrical Conductivity
Its electrical conductivity is notably high, often compared to that of copper. This property, combined with its chemical inertness, makes it an ideal material for electrodes.
Mechanical Hardness and Strength
With a hardness that approaches that of a diamond, glassy carbon is extremely durable and resistant to wear. This provides a stable and robust surface for both mechanical and electrochemical applications.
Key Considerations for Application
The properties of glassy carbon directly influence how it is used. The choice of specification is not just about size, but about how its inherent nature serves the intended purpose.
The Premier Electrode Material
Glassy carbon is a dominant material for working electrodes in electrochemistry. Its wide potential window, high conductivity, and chemical stability allow for the precise study of electrochemical reactions without interference from the electrode itself.
Surface Modifiability
The ability to easily polish, roughen, or apply coatings to the surface is a critical feature. Researchers can create a fresh, reproducible surface by polishing or functionalize it with specific coatings for developing advanced sensors and catalysts.
Structural Integrity and Gas Tightness
Glassy carbon has a low coefficient of thermal expansion, meaning it maintains its shape and size even with drastic temperature changes. Its structure also provides excellent gas tightness, a vital property for vacuum systems and sealed analytical cells.
Making the Right Choice for Your Application
Your final selection should be guided by the primary demands of your project.
- If your primary focus is electrochemical analysis: Select a standard thickness (e.g., 1mm or 2mm) and plan for surface polishing to ensure a clean, reproducible working area for your experiments.
- If your primary focus is high-temperature structural use: Choose a thicker sheet (e.g., 3mm) to maximize mechanical strength and thermal stability in furnaces or vacuum chambers.
- If your primary focus is advanced sensor development: A thinner sheet (e.g., 0.5mm or 1mm) may be suitable, but prioritize a supplier that offers well-defined options for surface roughening or coating.
Ultimately, aligning the material's inherent properties with your specific goal is the key to successful application.
Summary Table:
| Property | Specification |
|---|---|
| Standard Thickness | 0.5mm, 1mm, 2mm, 3mm |
| Standard Shape | Round, Square, Rectangular |
| Typical Dimensions | 5x5mm up to 100x100mm |
| Surface Finish | Polished, Roughened, Coated |
| Key Advantage | High thermal stability, chemical inertness, & electrical conductivity |
Ready to leverage the superior properties of glassy carbon in your laboratory?
KINTEK specializes in high-purity lab equipment and consumables, including precision glassy carbon sheets tailored for demanding applications in electrochemistry, high-temperature processing, and sensor development. Our materials ensure exceptional thermal stability (up to 3400°C), chemical inertness, and reliable performance.
Let us help you select the perfect specification for your project. Contact our experts today for a consultation and discover how KINTEK can enhance your research and production outcomes.
Visual Guide
Related Products
- Glassy Carbon Electrochemical Electrode
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Hydrophilic Carbon Paper TGPH060 for Battery Lab Applications
- Silicon Carbide (SIC) Ceramic Sheet Wear-Resistant Engineering Advanced Fine Ceramics
- Platinum Sheet Electrode for Laboratory and Industrial Applications
People Also Ask
- What is a glassy carbon electrode made of? The Engineered Material Powering Electrochemical Analysis
- How to make a glassy carbon electrode? A Guide to the Industrial Pyrolysis Process
- Why is a glassy carbon disc electrode an indispensable consumable? Ensure Reliable Catalyst Evaluation Today
- What are the pre-treatment steps for a glassy carbon electrode before use? Ensure Reliable Electrochemical Data
- How should a glassy carbon electrode be stored during long periods of non-use? Ensure Peak Performance & Longevity



















