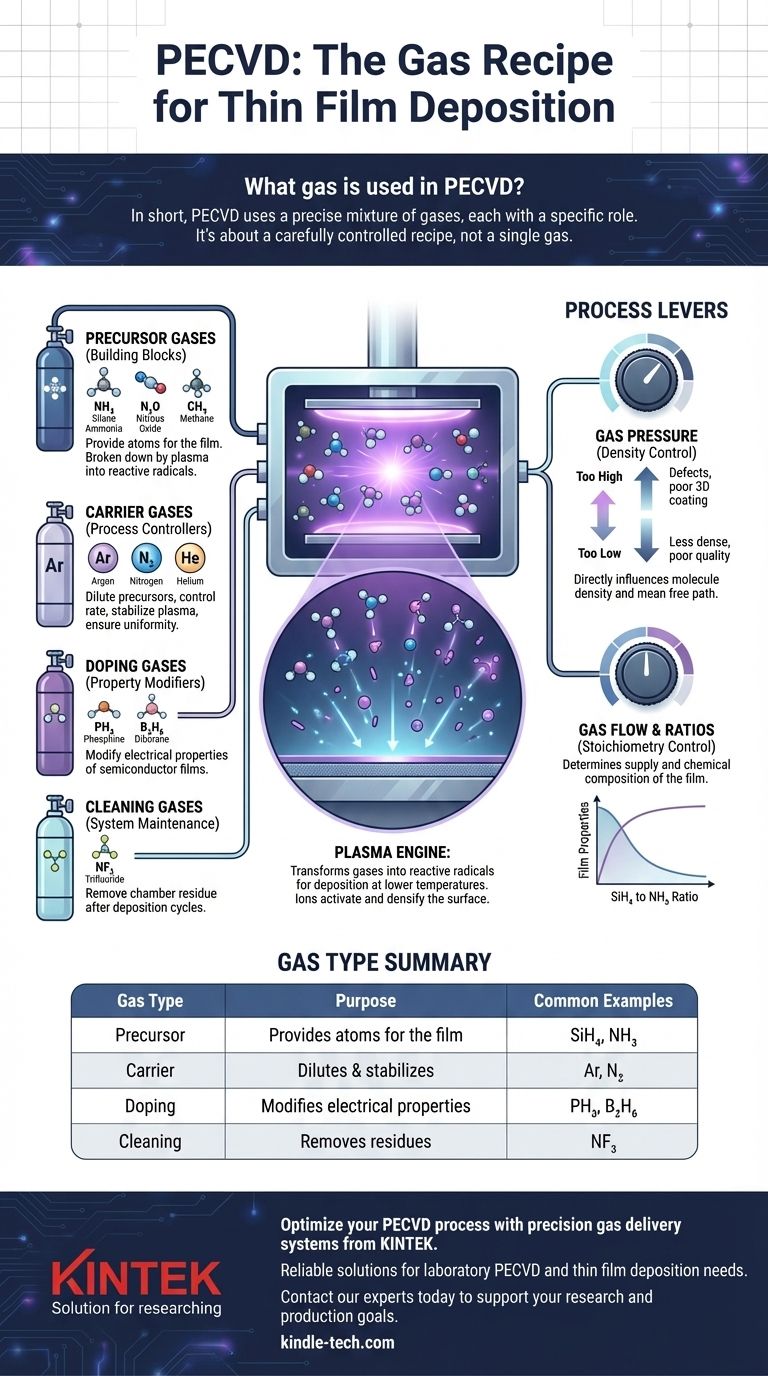
In short, PECVD uses a precise mixture of gases, each with a specific role. The primary gases are precursor gases like silane (SiH₄) and ammonia (NH₃) which contain the atoms for the film, and carrier gases like argon (Ar) or nitrogen (N₂) which are used to dilute the precursors and control the reaction. Other gases can be added for doping or chamber cleaning.
The key takeaway is that PECVD is not about a single gas, but about a carefully controlled recipe. The combination of precursor, carrier, and sometimes doping gases—activated by plasma—is what allows engineers to deposit high-quality thin films at significantly lower temperatures than traditional methods.

The Core Components of a PECVD Gas Mixture
The gas recipe in a Plasma-Enhanced Chemical Vapor Deposition (PECVD) process is fundamental to the properties of the final thin film. The gases can be categorized into several distinct functional groups.
Precursor Gases: The Building Blocks
Precursor gases are the essential ingredients that contain the atomic elements you intend to deposit. The plasma breaks these molecules down, allowing the desired atoms to settle on the substrate surface.
Common examples include:
- Silane (SiH₄): The primary source for depositing silicon (Si).
- Ammonia (NH₃): A common source of nitrogen (N) for silicon nitride (SiNₓ) films.
- Nitrous Oxide (N₂O): A source of oxygen (O) for silicon dioxide (SiO₂) films.
- Methane (CH₄): A source of carbon (C) for diamond-like carbon (DLC) films.
Carrier Gases: The Process Controllers
Carrier gases, also known as diluent gases, are inert and do not become part of the final film. Their purpose is to manage the deposition process.
They serve to dilute the reactive precursor gases, which helps control the deposition rate and ensure the reaction doesn't happen too quickly or uncontrollably. They also help stabilize the plasma and ensure an even distribution of reactive species over the substrate, leading to a more uniform film.
The most common carrier gases are Argon (Ar), Nitrogen (N₂), and Helium (He).
Doping Gases: Modifying Electrical Properties
In semiconductor manufacturing, it is often necessary to intentionally introduce impurities into a film to change its electrical characteristics. This is achieved by adding a small, precisely controlled amount of a doping gas to the main mixture.
Examples include phosphine (PH₃) for n-type doping (adding phosphorus) or diborane (B₂H₆) for p-type doping (adding boron).
Cleaning Gases: System Maintenance
After deposition cycles, residual material can build up inside the reaction chamber. To ensure process consistency, the chamber is periodically cleaned using a plasma process with a specific cleaning gas.
Gases like Nitrogen Trifluoride (NF₃) are highly effective at creating reactive fluorine radicals in the plasma, which etch away unwanted silicon-based residues from the chamber walls.
How Plasma Transforms These Gases
The "plasma" in PECVD is the engine that makes the process work. It is a highly energized state of gas, created by applying an electric field (typically radio frequency), which fundamentally changes how the gas molecules interact.
Creating Reactive Radicals
The immense energy in the plasma, primarily from free electrons, collides with the stable precursor gas molecules. These collisions are energetic enough to break chemical bonds, creating highly reactive molecular fragments known as radicals.
These radicals are the true agents of deposition. Because they are so reactive, they readily bond to the substrate surface to form the desired film, a process that would otherwise require extreme heat.
Surface Activation and Densification
The plasma also contains ions. These charged particles are accelerated by the electric field and bombard the surface of the growing film.
This ion bombardment serves two purposes. First, it activates the surface by creating available bonding sites (dangling bonds). Second, it physically compacts the deposited material, densifying the film and improving its overall quality and durability.
Understanding the Trade-offs: Gas Pressure and Flow
Achieving the desired film properties is a balancing act, and gas pressure and flow are two of the most critical control levers.
The Impact of Gas Pressure
Gas pressure directly influences the density of molecules in the chamber. Setting the right pressure is a crucial trade-off.
- Too high pressure: This increases the deposition rate but reduces the average distance a particle can travel before a collision (the "mean free path"). This is detrimental to coating complex, 3D structures and can lead to defects.
- Too low pressure: This can lead to a less dense, lower-quality film. The deposition mechanism itself can be altered, sometimes resulting in undesirable film structures.
The Importance of Gas Flow and Ratios
The absolute flow rate of each gas, managed by mass flow controllers, determines the supply of reactants. Just as important is the ratio between the different gases.
Changing the ratio of silane to ammonia, for instance, will directly alter the stoichiometry and refractive index of a silicon nitride film. This precise control is what makes PECVD such a powerful tool for engineering materials with specific properties.
Choosing the Right Gas Mix for Your Film
The selection of gases is dictated entirely by the desired properties of the final thin film. Your approach should be tailored to your specific goal.
- If your primary focus is depositing silicon nitride (SiNₓ): Your core recipe will be a silicon precursor like SiH₄ mixed with a nitrogen source like NH₃, often diluted with N₂.
- If your primary focus is depositing silicon dioxide (SiO₂): You will combine a silicon precursor like SiH₄ with an oxygen source, most commonly N₂O, along with a carrier gas.
- If your primary focus is controlling film quality and uniformity: You must add an inert carrier gas like Ar or N₂ to your mix to stabilize the plasma and ensure even deposition.
- If your primary focus is creating a doped semiconductor film: You will introduce a small, precisely metered amount of a doping gas like PH₃ or B₂H₆ into your main gas mixture.
Ultimately, mastering PECVD is about understanding how to use a specific gas recipe to translate plasma chemistry into a functional, high-quality material.
Summary Table:
| Gas Type | Purpose | Common Examples |
|---|---|---|
| Precursor | Provides atoms for the film | Silane (SiH₄), Ammonia (NH₃) |
| Carrier | Dilutes precursors & stabilizes plasma | Argon (Ar), Nitrogen (N₂) |
| Doping | Modifies electrical properties | Phosphine (PH₃), Diborane (B₂H₆) |
| Cleaning | Removes chamber residues | Nitrogen Trifluoride (NF₃) |
Optimize your PECVD process with precision gas delivery systems from KINTEK.
Whether you are depositing silicon nitride, silicon dioxide, or doped semiconductor films, the right gas mixture is critical for achieving high-quality, uniform thin films at lower temperatures. KINTEK specializes in lab equipment and consumables, providing reliable solutions for your laboratory's PECVD and thin film deposition needs.
Contact our experts today to discuss how we can support your research and production goals with tailored equipment and consumables.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Vertical Laboratory Tube Furnace
People Also Ask
- What are the process capabilities of ICPCVD systems? Achieve Low-Damage Film Deposition at Ultra-Low Temperatures
- What is the difference between plasma CVD and thermal CVD? Choose the Right Method for Your Substrate
- How do PECVD systems improve DLC coatings on implants? Superior Durability and Biocompatibility Explained
- Why is a Matching Network Indispensable in RF-PECVD for Siloxane Films? Ensure Stable Plasma and Uniform Deposition
- What is the process of PECVD in semiconductor? Enabling Low-Temperature Thin Film Deposition



















