At its core, hardening is a heat treatment process that increases the hardness of steel. It involves heating the metal to a very high temperature and then cooling it rapidly, a procedure known as quenching. This process fundamentally changes the steel's internal structure to make it stronger and more resistant to wear.
The goal of hardening is not simply to heat and cool steel, but to rapidly transform its crystal structure into a highly stressed, hard state called martensite, effectively locking its atoms into a configuration that resists deformation.

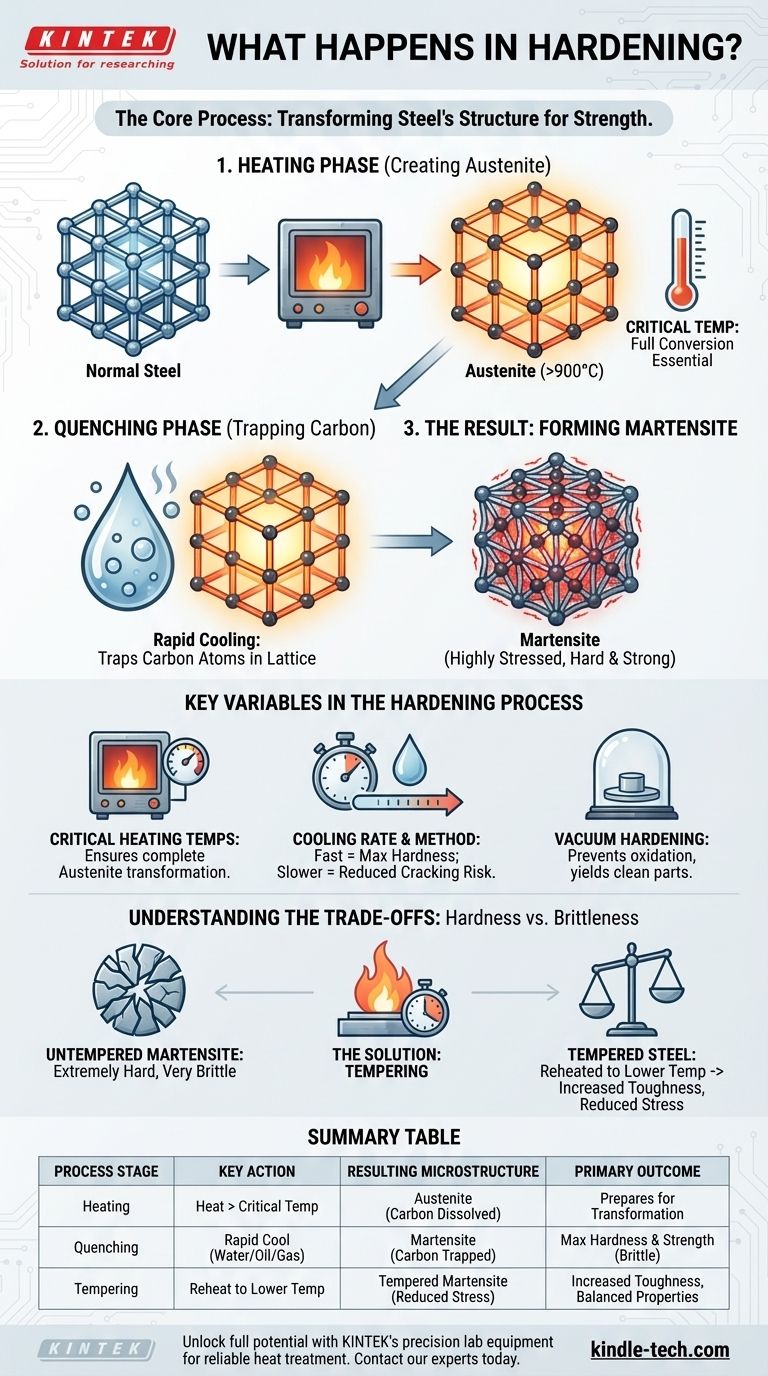
The Core Mechanism: Transforming Steel's Crystal Structure
To truly understand what happens during hardening, you must look at the atomic level. The process is a carefully controlled manipulation of steel's crystalline lattice.
The Heating Phase: Creating Austenite
When steel is heated above its critical temperature (typically over 900°C), its internal crystal structure rearranges. It transforms into a phase called austenite.
The key property of austenite is its ability to absorb carbon atoms from the steel into its crystal lattice. This even distribution of carbon is the essential first step.
The Quenching Phase: Trapping Carbon
The second step, quenching, involves cooling the steel so rapidly that the carbon atoms do not have time to move out of the crystal structure as they normally would during slow cooling.
Common quenching mediums include water, oil, brine, or inert gases like nitrogen, with the choice depending on the type of steel and the desired cooling speed.
The Result: Forming Martensite
This rapid cooling forces the austenite to transform into a new, highly strained crystal structure called martensite.
Because the carbon atoms are trapped within the lattice, the structure is put under immense internal stress. This stressed state is what makes martensite—and thus the hardened steel—extremely hard and strong.
Key Variables in the Hardening Process
The final properties of the steel are not accidental; they are the direct result of controlling several critical variables during the heat treatment.
Critical Heating Temperatures
Heating the steel to the correct temperature is non-negotiable. The goal is to fully convert the material to austenite. Insufficient heat results in an incomplete transformation and a less effective hardening process.
Cooling Rate and Quench Method
The speed of cooling determines the final outcome. A very fast quench (e.g., in water or brine) maximizes the formation of martensite and achieves the highest possible hardness.
A slower quench (e.g., in oil or gas) is used for certain steel alloys to reduce the risk of cracking or distortion while still achieving significant hardness.
Specialized Environments: Vacuum Hardening
As noted in advanced applications, this process can be done in a vacuum furnace. The primary benefit of vacuum hardening is to prevent surface reactions like oxidation, resulting in a clean, scale-free part that requires less finishing work.
Understanding the Trade-offs: Hardness vs. Brittleness
Achieving maximum hardness comes at a cost. Understanding this trade-off is critical for any practical application.
The Inherent Brittleness of Martensite
While the new martensitic structure is incredibly hard, it is also very brittle. A fully hardened, untempered piece of steel is often too brittle for practical use and can shatter like glass under sharp impact.
The Solution: Tempering
To solve this, a secondary heat treatment called tempering is almost always performed after hardening. The part is reheated to a much lower temperature and held for a specific time.
This process relieves some of the internal stress within the martensite, trading a small amount of hardness for a significant increase in toughness—the ability to absorb energy and resist fracture.
Making the Right Choice for Your Goal
The specific parameters of the hardening and tempering processes are selected based on the final requirements of the component.
- If your primary focus is maximum wear resistance and surface hardness: You need a process that creates a high percentage of martensite, often achieved with the fastest possible quench the material can tolerate without cracking.
- If your primary focus is balanced strength and toughness for stressed parts: You need a hardening process followed by a precise tempering cycle to reduce brittleness to an acceptable level for the application.
Ultimately, hardening is the foundational process for unlocking the full performance potential of steel.
Summary Table:
| Process Stage | Key Action | Resulting Microstructure | Primary Outcome |
|---|---|---|---|
| Heating | Heat steel above critical temperature (e.g., 900°C) | Austenite (carbon is dissolved in the lattice) | Prepares steel for transformation |
| Quenching | Rapidly cool steel in water, oil, or gas | Martensite (carbon is trapped, creating internal stress) | Maximum hardness and strength, but high brittleness |
| Tempering | Reheat to a lower temperature and hold | Tempered Martensite (reduced internal stress) | Increased toughness and ductility, balanced properties |
Unlock the full potential of your materials with KINTEK's precision lab equipment.
Hardening is a delicate science, and achieving the perfect balance of hardness and toughness requires reliable, consistent heat treatment. KINTEK specializes in high-performance laboratory furnaces and quenching systems designed for exacting processes like vacuum hardening, which prevents oxidation and delivers clean, scale-free results.
Whether you are developing cutting tools, automotive components, or any part requiring superior wear resistance, our equipment ensures precise temperature control and repeatability for trustworthy outcomes every time.
Ready to enhance your lab's capabilities and achieve superior material performance? Contact our experts today via our Contact Form to discuss your specific hardening application and discover the ideal KINTEK solution for your laboratory needs.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- Vertical Laboratory Tube Furnace
People Also Ask
- What is vacuum heat treatment? Achieve Superior Material Performance and Pristine Finishes
- What is 1800 degrees Celsius furnace? A Guide to High-Temperature Materials Processing
- What is the temperature and holding time for sintering? Master the Variables for Optimal Results
- What are the products of pyrolysis combustion? Unlocking Valuable Biochar, Bio-oil, and Syngas
- How hot does a furnace heat exchanger get? Understand Safe Operating Temperatures to Prevent Hazards
- What are powder sintering methods? A Guide to Metal & Ceramic Part Manufacturing
- What is the vacuum heat treatment process? Achieve Purity and Precision for High-Performance Metals
- What is the temperature of the arc in an electric arc furnace? Harnessing Heat Hotter Than the Sun



















