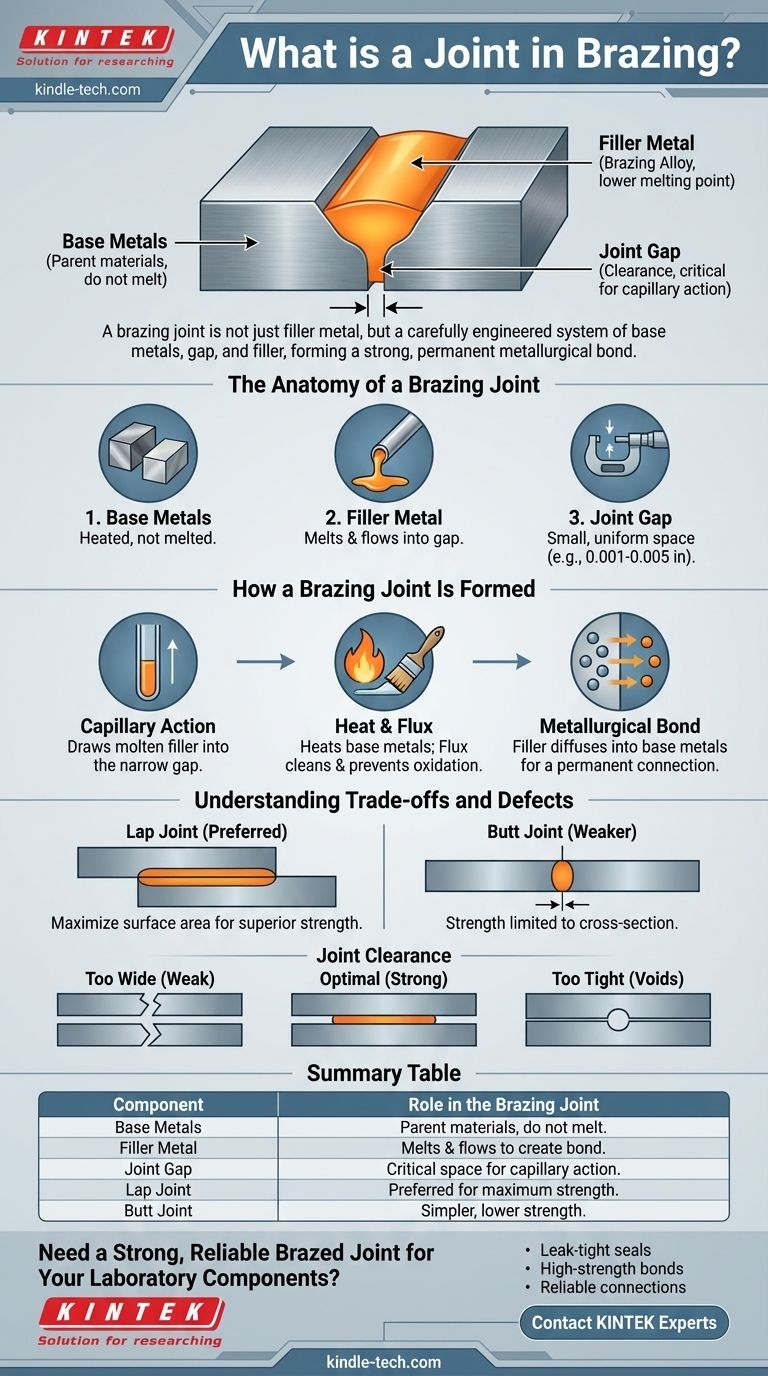
In brazing, a joint is the entire region where two or more metal components are bonded together using a molten filler metal. The joint is not just the filler metal itself, but a complete system that includes the surfaces of the base metals being joined and the gap between them, into which the filler metal is drawn by capillary action to create a strong, permanent bond.
The key to understanding a brazing joint is to see it not as a simple adhesive connection, but as a carefully engineered metallurgical system. The strength of the joint is determined less by the strength of the filler metal alone and more by the design of the joint, particularly the tight clearance between the parts.

The Anatomy of a Brazing Joint
A successful brazing joint is composed of three essential elements working in concert. Understanding each one is critical to controlling the outcome.
The Base Metals
These are the parent materials you are intending to join. The brazing process is designed so that these metals are heated, but they do not melt.
The Filler Metal (Brazing Alloy)
This is a metal or alloy with a melting point that is lower than the base metals. When it becomes molten, its properties allow it to flow into the gap between the base metals.
The Joint Gap (Clearance)
This is the most critical design factor in a brazing joint. The joint clearance is the small, uniform space between the base metals that the filler metal will occupy. This gap is precisely what enables the core principle of brazing.
How a Brazing Joint Is Formed
The formation of a joint is a physical and chemical process that relies on heat, cleanliness, and a phenomenon known as capillary action.
The Power of Capillary Action
Capillary action is the primary force that distributes the filler metal throughout the joint. Just as a paper towel draws water into its fibers, the narrow joint clearance pulls the molten filler metal into the gap, even against the force of gravity.
This action ensures the entire joint area is filled with alloy, creating a complete and uniform connection.
The Role of Heat and Flux
The base metals are heated to a temperature above the melting point of the filler metal. This heat allows the filler to melt and flow when it is introduced.
For capillary action to work, the surfaces must be perfectly clean. A flux is a chemical compound applied to the joint area that prevents oxidation during heating and cleans the surfaces, allowing the filler metal to "wet" and flow freely across the base metals.
Creating a Metallurgical Bond
As the filler metal cools and solidifies, it forms a metallurgical bond with the base metals. This is not a simple mechanical bond; atoms from the filler metal diffuse into the surface of the base metals (and vice-versa), creating a strong, permanent, and often leak-tight connection.
Understanding the Trade-offs and Defects
The design of the joint directly dictates its strength and reliability. Poor design leads to predictable failures.
Lap Joints vs. Butt Joints
The two most common designs are the lap joint and the butt joint.
A butt joint joins two surfaces end-to-end. Its strength is limited to the cross-sectional area of the thinnest part, making it weaker.
A lap joint, where one part overlaps the other, is almost always preferred for brazing. This design increases the surface area for bonding, and the strength of the joint can easily be made to exceed the strength of the base metals themselves.
The Critical Role of Clearance
The joint clearance is a trade-off. If the gap is too wide, capillary action will fail, and the joint will be weak because its strength will be limited to that of the filler metal alone.
If the gap is too tight, the filler metal cannot flow into the joint at all, resulting in voids and no bond. Optimal clearance for most alloys is between 0.001 and 0.005 inches (0.025 mm to 0.127 mm).
The Consequence of Joint Defects
A defect occurs when the joint is not properly formed, often due to poor cleaning, incorrect clearance, or improper heating. This can create voids where the filler alloy did not flow.
When a defect is found, it can often be repaired. However, simply reheating the part is not advisable. After the initial braze cycle, most filler alloys develop a higher remelt temperature, making it difficult to rework the existing alloy. Applying a small amount of new filler to the defective area is the more reliable repair method.
Making the Right Choice for Your Goal
Achieving a sound joint requires designing it for the forces it will encounter and the process you are using.
- If your primary focus is maximum strength: Design a lap joint with an overlap three to four times the thickness of the thinnest base metal and maintain a strict joint clearance.
- If your primary focus is avoiding defects: Prioritize meticulous cleaning of the base metals and ensure proper application of flux or use of a controlled atmosphere to guarantee the filler alloy can wet and flow.
- If your primary focus is repairing a faulty joint: Do not simply reheat the assembly; add a small amount of new filler alloy to the specific defect to ensure a proper fill and bond.
Ultimately, a well-designed brazing joint is a testament to the principle that the whole is stronger than the sum of its parts.
Summary Table:
| Component | Role in the Brazing Joint |
|---|---|
| Base Metals | The parent materials being joined; they do not melt. |
| Filler Metal | The alloy that melts and flows into the joint gap to create the bond. |
| Joint Gap (Clearance) | The critical, precise space (0.001-0.005 in) that enables capillary action. |
| Lap Joint | Preferred design for maximum strength, using an overlap for greater bonding area. |
| Butt Joint | A simpler, end-to-end design with lower strength than a lap joint. |
Need a Strong, Reliable Brazed Joint for Your Laboratory Components?
A successful brazing process is fundamental to the performance and longevity of your lab equipment. At KINTEK, we specialize in providing the high-quality materials and expert support needed for flawless brazing results.
We help you achieve:
- Leak-tight seals for vacuum systems and fluid paths.
- High-strength bonds that can withstand thermal cycling and mechanical stress.
- Reliable connections for custom fixtures, heating elements, and instrument assemblies.
Whether you're working on a prototype or scaling up production, our team can assist with selecting the right filler metals and fluxes for your specific base metals and application requirements.
Contact our brazing experts today to discuss how we can support your laboratory's fabrication and repair needs.
Visual Guide
Related Products
- Vacuum Bellows for Efficient Connection and Stable Vacuum in High-Performance Systems
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
- Electrode Polishing Material for Electrochemical Experiments
People Also Ask
- How can a gas ballast valve be used as a diagnostic tool? Identify Oil Contamination vs. System Leaks
- What environmental protection do mechanical vacuum pump sets provide during zirconium alloy melting? Prevent Embrittlement
- What is the function of a laboratory vacuum system in preparing COF precursors? Ensure Purity & Prevent Oxidation
- Why are specialized vacuum sealing components necessary for transferring high-purity salt samples? Ensure Data Integrity
- What material is used for furnace heating? Select the Right Element for Your Process






