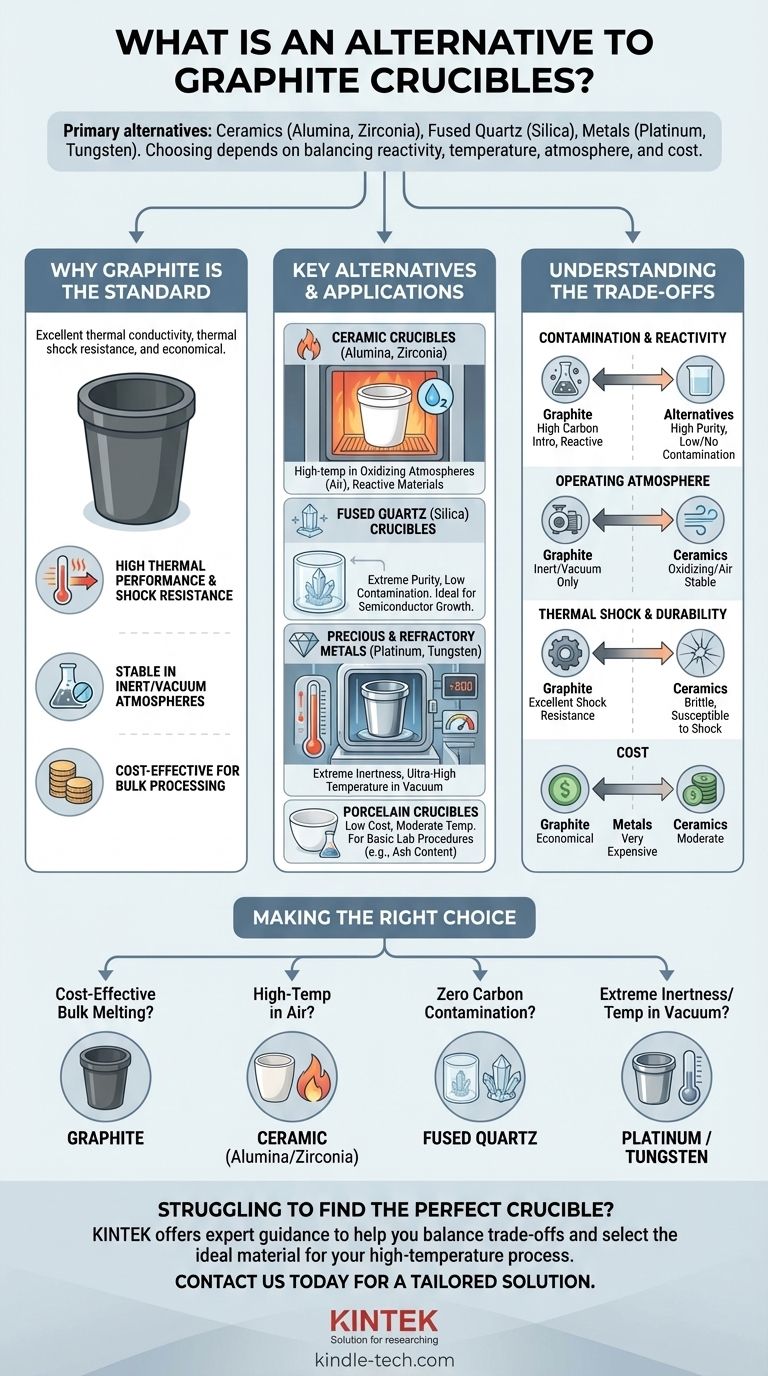
The primary alternatives to graphite crucibles are materials chosen for specific needs like higher purity or resistance to oxidation, including ceramics like alumina and zirconia, fused quartz (silica), and various metals such as platinum or tungsten. While graphite is a versatile and cost-effective standard for melting many metals, it is not universally suitable for every high-temperature application.
Choosing a crucible is not about finding a universal "best" material, but about understanding the critical trade-offs between chemical reactivity, operating temperature, atmosphere, and cost for your specific process.

Why Graphite Is the Standard
Before exploring alternatives, it's essential to understand why graphite is so widely used. Its properties make it a default choice for many applications, from foundries to laboratories.
High Thermal Performance
Graphite offers excellent thermal conductivity, allowing for rapid and uniform heating of the material inside.
It also has exceptional thermal shock resistance, meaning it can withstand rapid temperature changes without cracking—a common failure point for more brittle materials.
Chemical Properties
Graphite is highly effective for smelting and melting most non-ferrous metals and their alloys. It is chemically stable in inert or vacuum atmospheres even at very high temperatures.
Cost-Effectiveness
As noted in many industrial applications, graphite provides a balance of high performance and low cost. This makes it an economical choice for bulk metal processing and general-purpose use.
Key Alternatives and Their Applications
When the limitations of graphite become a factor, engineers and scientists turn to a range of specialized materials.
Ceramic Crucibles (Alumina, Zirconia)
Ceramic crucibles are a leading alternative when operating in an oxidizing atmosphere (air) at high temperatures, where graphite would burn away.
Alumina (Al₂O₃) is a cost-effective ceramic choice for high-purity applications up to about 1700°C. Zirconia (ZrO₂) is used for even higher temperatures and when melting highly reactive materials.
Fused Quartz (Silica) Crucibles
Fused quartz is essentially high-purity glass. Its primary advantage is its extremely low potential for contamination.
It is the ideal choice for growing semiconductor crystals or when any carbon contamination from a graphite crucible would compromise the final product.
Porcelain Crucibles
Porcelain is a low-cost ceramic used for basic laboratory procedures. It's suitable for applications like determining the ash content of a sample, where temperatures are more moderate and chemical inertness is less critical than with reactive metals.
Precious and Refractory Metal Crucibles
For the most demanding conditions, crucibles are made from metals with extremely high melting points.
Platinum is used for its exceptional chemical inertness, especially in analytical chemistry or when melting high-purity glass. Tungsten and Molybdenum are used in vacuum furnaces for applications requiring temperatures far exceeding the limits of ceramics.
Understanding the Trade-offs
Selecting an alternative to graphite requires a careful evaluation of three key factors. No material is perfect; each comes with compromises.
Contamination and Reactivity
Graphite can react with certain metals to form carbides and will introduce carbon into the melt, which can be undesirable. Ceramic and quartz crucibles offer a significant advantage in purity.
Operating Atmosphere
This is often the deciding factor. Graphite excels in vacuum or inert gas, but it will rapidly oxidize and degrade in an air furnace at high temperatures. Ceramics are the opposite; they are stable in air but may be less suitable for hard vacuum environments.
Thermal Shock and Durability
Graphite's ability to handle rapid heating and cooling is its greatest mechanical strength. Ceramics are brittle and can easily crack if heated or cooled too quickly, requiring carefully controlled furnace cycles.
Cost
Graphite is almost always the most economical option. Ceramic crucibles are moderately more expensive, while crucibles made of platinum or tungsten are orders of magnitude more costly and reserved for highly specialized work.
Making the Right Choice for Your Application
To select the correct crucible, start by defining your most critical process requirement.
- If your primary focus is cost-effective bulk metal melting: Graphite remains the industry standard for its durability and low cost.
- If your primary focus is high-temperature work in an air furnace: An alumina or zirconia ceramic crucible is the correct choice.
- If your primary focus is preventing all carbon contamination: Fused quartz (silica) provides the necessary purity for sensitive materials.
- If your primary focus is extreme chemical inertness or ultra-high temperatures in a vacuum: A platinum or tungsten crucible is required.
Ultimately, selecting the right crucible is a matter of matching the material's unique properties to the precise demands of your process.
Summary Table:
| Alternative Material | Key Properties | Ideal Applications |
|---|---|---|
| Ceramic (Alumina/Zirconia) | High-temperature stability in air, good purity | Melting in oxidizing atmospheres, reactive materials |
| Fused Quartz (Silica) | Extreme purity, low contamination | Semiconductor crystal growth, carbon-sensitive processes |
| Precious/Refractory Metal (Pt, W) | Extreme inertness, ultra-high temperature | High-purity glass melting, demanding vacuum furnace applications |
| Porcelain | Low cost, moderate temperature resistance | Basic lab procedures (e.g., ash content determination) |
Struggling to find the perfect crucible for your specific application? The right choice is critical for the success of your high-temperature process. KINTEK specializes in lab equipment and consumables, offering expert guidance to help you select the ideal crucible material—whether you need the cost-effectiveness of graphite, the high purity of quartz, or the extreme performance of ceramics and metals. Our team can help you balance the trade-offs between temperature, atmosphere, reactivity, and cost to ensure optimal results.
Contact us today via our [#ContactForm] to discuss your requirements and let our specialists provide a solution tailored to your laboratory's unique needs.
Visual Guide
Related Products
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- High Purity Pure Graphite Crucible for Evaporation
- High Purity Pure Graphite Crucible for Electron Beam Evaporation
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Custom Machined and Molded PTFE Teflon Parts Manufacturer with PTFE Crucible and Lid
People Also Ask
- How does the use of corrosion-resistant ceramic crucibles ensure the chemical purity of materials? | KINTEK
- What is a crucible material for a furnace? A Guide to Choosing the Right High-Temperature Container
- What role does an Alumina Crucible play in the high-temperature solid-state synthesis of Na3OBr? Ensure Sample Purity
- What is the function of an alumina crucible in NaSICON synthesis? Ensure Purity in High-Temperature Reactions
- Why are alumina crucibles or baskets essential for Boudouard reaction studies? Ensure Pure Data & Chemical Inertness



















