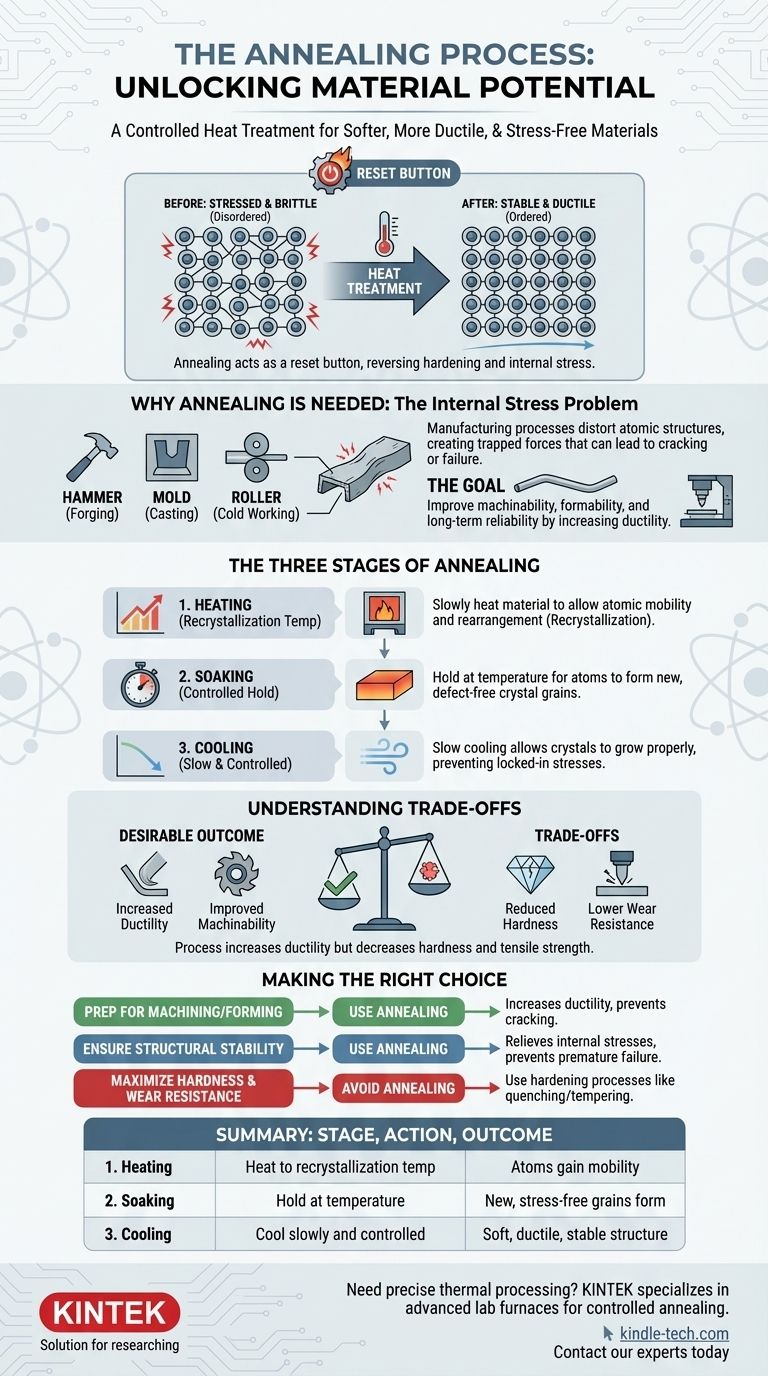
In essence, annealing is a heat treatment process used to make a material, typically a metal, softer, more ductile (easier to shape), and to relieve internal stresses. It involves heating the material to a specific temperature, holding it there for a period, and then cooling it at a controlled, slow rate. This process effectively resets the material's internal structure, making it more uniform and workable.
Annealing acts as a "reset button" for a material's internal structure. It reverses the hardening and stress introduced during manufacturing, transforming a brittle, stressed material into a stable, ductile, and more easily workable one.

Why Materials Need Annealing: The Problem of Internal Stress
When metals are manufactured through processes like casting (pouring molten metal into a mold), forging (hammering into shape), or cold working (bending or rolling at room temperature), their internal crystalline structures become distorted and stressed.
The Source of Hardness and Stress
Think of the atoms in a metal as being arranged in a neat, orderly grid. Processes like bending or hammering force these atoms out of alignment, creating defects and tangles in the grid.
This disordered state makes the material harder and more brittle. It also creates internal stresses—forces trapped within the material that are constantly pulling and pushing on its internal structure.
The Dangers of Internal Stress
Internal stresses are a hidden risk. A component might look perfectly fine, but these internal forces can lead to spontaneous cracking or failure over time, especially when subjected to vibration or temperature changes.
Annealing is the primary method for relieving these dangerous internal stresses, significantly improving the long-term reliability of a component.
The Goal: Improving Workability
A hard, brittle material is difficult to machine, bend, or shape without it cracking. By reducing hardness and increasing ductility (the ability to deform without breaking), annealing makes subsequent manufacturing steps much easier and more predictable.
The Three Stages of the Annealing Process
Annealing is not simply heating and cooling; it is a precise, three-stage process that carefully manipulates the material's atomic structure.
Stage 1: Heating to the Recrystallization Temperature
First, the material is slowly heated to a specific temperature. This temperature is critical—it's hot enough to allow the atoms within the crystal structure to move and rearrange, a process called recrystallization.
Crucially, the material remains in a solid state. The energy from the heat simply gives the atoms enough mobility to "un-tangle" themselves from the stressed positions they were forced into during fabrication.
Stage 2: Soaking at a Controlled Temperature
Once the target temperature is reached, the material is "soaked" or held at that temperature for a set amount of time. This holding period gives the atoms sufficient time to form new, defect-free, and stress-free crystal grains. The longer the soak, the more uniform the resulting structure becomes.
Stage 3: Slow and Controlled Cooling
This is arguably the most critical stage. The material must be cooled very slowly. If it were cooled too quickly (a process known as quenching), the stresses would be locked back into the structure, defeating the purpose of annealing.
Slow cooling allows the newly formed, orderly crystals to grow properly, resulting in a soft, ductile, and internally stable microstructure.
Understanding the Trade-offs
While highly beneficial, annealing is a tool for a specific purpose, and its effects involve clear trade-offs. The primary outcome is a softer, more ductile material.
Reduced Hardness and Strength
Annealing intentionally reduces a material's hardness and tensile strength. This is desirable for improving machinability and formability, but it makes the final product less resistant to wear, abrasion, and deformation under load.
Increased Ductility vs. Wear Resistance
The process creates a trade-off between ductility and hardness. An annealed part is easy to bend and shape but will wear down more quickly than a hardened part. The choice depends entirely on the component's final application.
Making the Right Choice for Your Goal
Applying annealing depends entirely on what you need to achieve with your material.
- If your primary focus is preparing a material for machining or cold working: Annealing is an essential step to increase ductility and prevent the material from cracking during fabrication.
- If your primary focus is to ensure long-term structural stability: Annealing is critical for relieving internal stresses induced by processes like welding or casting, preventing premature failure.
- If your primary focus is to maximize hardness and wear resistance for a final product: Annealing is the opposite of what you need; you would instead use a hardening process like quenching and tempering.
Ultimately, annealing provides precise control over a material's internal state, transforming it from stressed and brittle to stable and workable.
Summary Table:
| Annealing Stage | Key Action | Primary Outcome |
|---|---|---|
| 1. Heating | Heat to recrystallization temperature | Atoms gain mobility to rearrange |
| 2. Soaking | Hold at temperature | New, stress-free crystal grains form |
| 3. Cooling | Cool slowly and controlled | Soft, ductile, and stable structure is achieved |
Need precise thermal processing for your materials?
KINTEK specializes in advanced lab equipment, including furnaces ideal for controlled annealing processes. Whether you're developing new alloys, preparing samples for testing, or ensuring the reliability of your components, our solutions deliver the uniform heating and precise temperature control essential for successful results.
Contact our experts today to discuss how we can support your laboratory's material science and heat treatment needs.
Visual Guide
Related Products
- Vertical Laboratory Tube Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
People Also Ask
- What temperature is tube annealing? A Guide to Material-Specific Ranges for Optimal Results
- Why does heating increase temperature? Understanding the Molecular Dance of Energy Transfer
- What is a vertical tube furnace? Leverage Gravity for Superior Uniformity and Process Control
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost
- What is the temperature of a quartz tube furnace? Master the Limits for Safe, High-Temp Operation



















