There is no single "best" electric heating element. The ideal choice is entirely dependent on your application's specific requirements. The most critical factors are the maximum operating temperature you need to achieve and the chemical atmosphere inside your furnace or oven.
The challenge is not to find a universally "best" material, but to correctly match the element's properties—primarily its temperature limit, atmospheric compatibility, and cost—to the precise demands of your heating process.

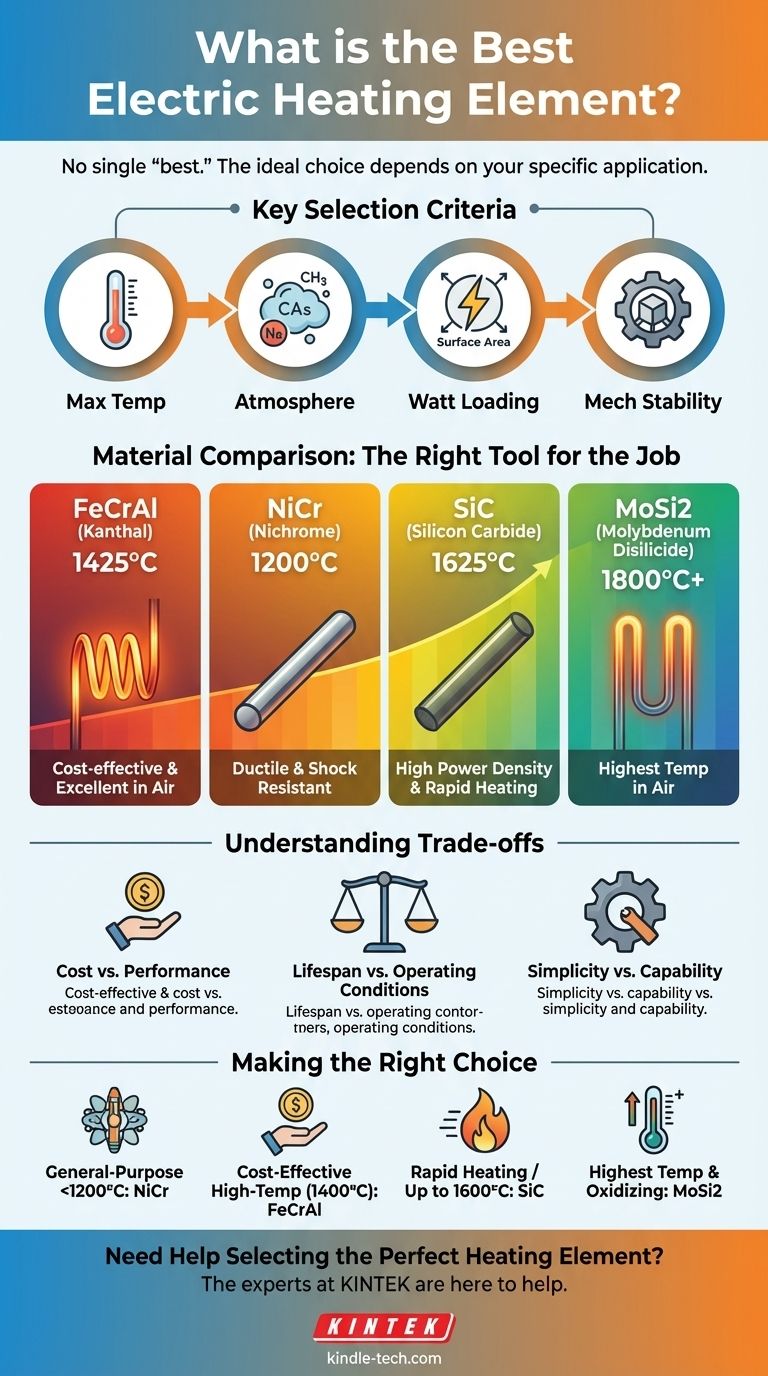
Key Selection Criteria for Heating Elements
Before comparing materials, you must first define your operational needs. The answers to these questions will quickly narrow your options from a wide field to a few suitable candidates.
Maximum Operating Temperature
This is the single most important factor. Each material has a firm upper limit beyond which it will rapidly degrade and fail. Always select an element with a maximum temperature rating comfortably above your intended process temperature.
Furnace Atmosphere
The gases surrounding the element are critical. An element that thrives in an oxidizing atmosphere (like air) may be quickly destroyed in a reducing atmosphere (like hydrogen or cracked ammonia), and vice versa.
Watt Loading
Watt loading refers to the power output per unit of the element's surface area. Aggressive, high watt loading allows for faster heat-up times but can significantly shorten the element's lifespan. Some materials can handle much higher watt loads than others.
Mechanical Stability
Heating elements must be mechanically supported. Some materials, like metallic alloys, are ductile and can be easily formed into coils. Others are brittle ceramics that are self-supporting but fragile. You must also consider whether the material will sag ("creep") at high temperatures over time.
A Comparison of Common Heating Element Materials
With your requirements defined, you can now evaluate the most common materials used in industrial and laboratory heating.
Iron-Chrome-Aluminum (FeCrAl / "Kanthal"): The Workhorse
FeCrAl alloys are the most widely used metallic heating elements. They form a stable, protective aluminum oxide (Al2O3) layer, which gives them excellent performance in air.
They are ideal for furnaces and kilns operating in oxidizing atmospheres up to approximately 1425°C (2600°F). They are also the most cost-effective option for high-temperature applications.
Nickel-Chrome (NiCr / "Nichrome"): The Ductile Choice
NiCr alloys are another extremely common choice, especially for applications below 1200°C (2190°F). Their key advantage is that they do not become brittle with use.
This high ductility makes them resistant to the vibration and mechanical shock that can fracture other elements. They are often used in applications where reliability and ease of forming are paramount.
Silicon Carbide (SiC): For High Power Density
SiC is a ceramic material that offers a significant step up in temperature capability, operating up to 1625°C (2957°F). It is structurally rigid and can be mounted as a self-supporting rod or U-shape.
These elements can handle very high watt loads, making them perfect for applications requiring rapid heating. However, their electrical resistance increases with age, requiring a more sophisticated power control system (like a tap transformer or an SCR) to compensate.
Molybdenum Disilicide (MoSi2): For the Highest Temperatures
For the most demanding applications in air, MoSi2 elements are the top choice, capable of reaching temperatures over 1800°C (3270°F). They form a protective silica glass layer that allows for this extreme performance.
They are extremely brittle at room temperature and are susceptible to a low-temperature oxidation known as "pest" if held for long periods between 400-700°C. They are the most expensive option but are necessary for specialized high-temperature processes like sintering ceramics.
Understanding the Trade-offs
Selecting an element always involves balancing competing factors. Being aware of these compromises is key to making a sound engineering decision.
Cost vs. Performance
There is a direct correlation between price and maximum operating temperature. A FeCrAl element is significantly less expensive than a MoSi2 element, but it simply cannot perform the same job. Over-specifying an element for a low-temperature job is a waste of money.
Lifespan vs. Operating Conditions
Consistently running an element at its absolute maximum rated temperature will drastically shorten its life. Operating it just 50-100°C below its limit can often double its service life. Similarly, exposing an element to a contaminant in the furnace atmosphere can lead to premature failure.
Simplicity vs. Capability
Metallic elements like FeCrAl and NiCr are simple to power with basic controls. High-performance ceramic elements like SiC and MoSi2 are more brittle, sensitive to thermal shock, and often require advanced power controllers to manage changes in their electrical resistance.
Making the Right Choice for Your Application
Use your primary goal as the final filter to select the optimal material.
- If your primary focus is general-purpose heating below 1200°C: NiCr offers excellent durability, ductility, and ease of use for a wide range of applications.
- If your primary focus is cost-effective, high-temperature heating in air (up to 1400°C): FeCrAl (Kanthal) is the undisputed industry standard for its performance and value.
- If your primary focus is rapid heating or temperatures up to 1600°C: SiC elements are a robust choice but require investment in appropriate power control systems.
- If your primary focus is reaching the highest possible temperatures in an oxidizing atmosphere: MoSi2 is the premium material, essential for specialized processes despite its cost and fragility.
By aligning the element's material properties with your specific operational demands, you ensure both performance and longevity for your process.
Summary Table:
| Material | Max Temperature | Key Advantage | Ideal For |
|---|---|---|---|
| Iron-Chrome-Aluminum (FeCrAl) | Up to 1425°C | Cost-effective, excellent in air | General-purpose high-temp heating in oxidizing atmospheres |
| Nickel-Chrome (NiCr) | Up to 1200°C | Highly ductile, resistant to shock | Applications requiring durability and ease of forming |
| Silicon Carbide (SiC) | Up to 1625°C | High power density, rapid heating | Fast heat-up cycles and high-temp processes |
| Molybdenum Disilicide (MoSi2) | Over 1800°C | Highest temperature capability in air | Specialized, extreme-temperature applications |
Need Help Selecting the Perfect Heating Element?
Choosing the wrong heating element can lead to premature failure, process inefficiency, and unexpected costs. The experts at KINTEK are here to help. We specialize in providing the right lab equipment and consumables for your specific thermal processing needs.
We will help you:
- Analyze your application's requirements for temperature, atmosphere, and watt loading.
- Recommend the optimal element material to maximize performance and lifespan.
- Source reliable components from leading manufacturers.
Don't leave your process to chance. Contact our technical team today for a personalized consultation and ensure your furnace operates at peak efficiency.
Contact KINTEK for Expert Guidance
Visual Guide
Related Products
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Automatic Laboratory Heat Press Machine
- Double Plate Heating Press Mold for Lab
- Infrared Heating Quantitative Flat Plate Press Mold
People Also Ask
- Which high temperature furnace elements to be used in oxidizing atmosphere? MoSi2 or SiC for Superior Performance
- What is the thermal expansion coefficient of molybdenum disilicide? Understanding its role in high-temperature design
- What is the temperature range of molybdenum disilicide heating elements? Choose the Right Grade for Your High-Temp Needs
- What are the properties of molybdenum heating element? Choose the Right Type for Your Furnace Atmosphere
- What is the temperature range of a MoSi2 heating element? Unlock 1900°C Performance for Your Lab
















