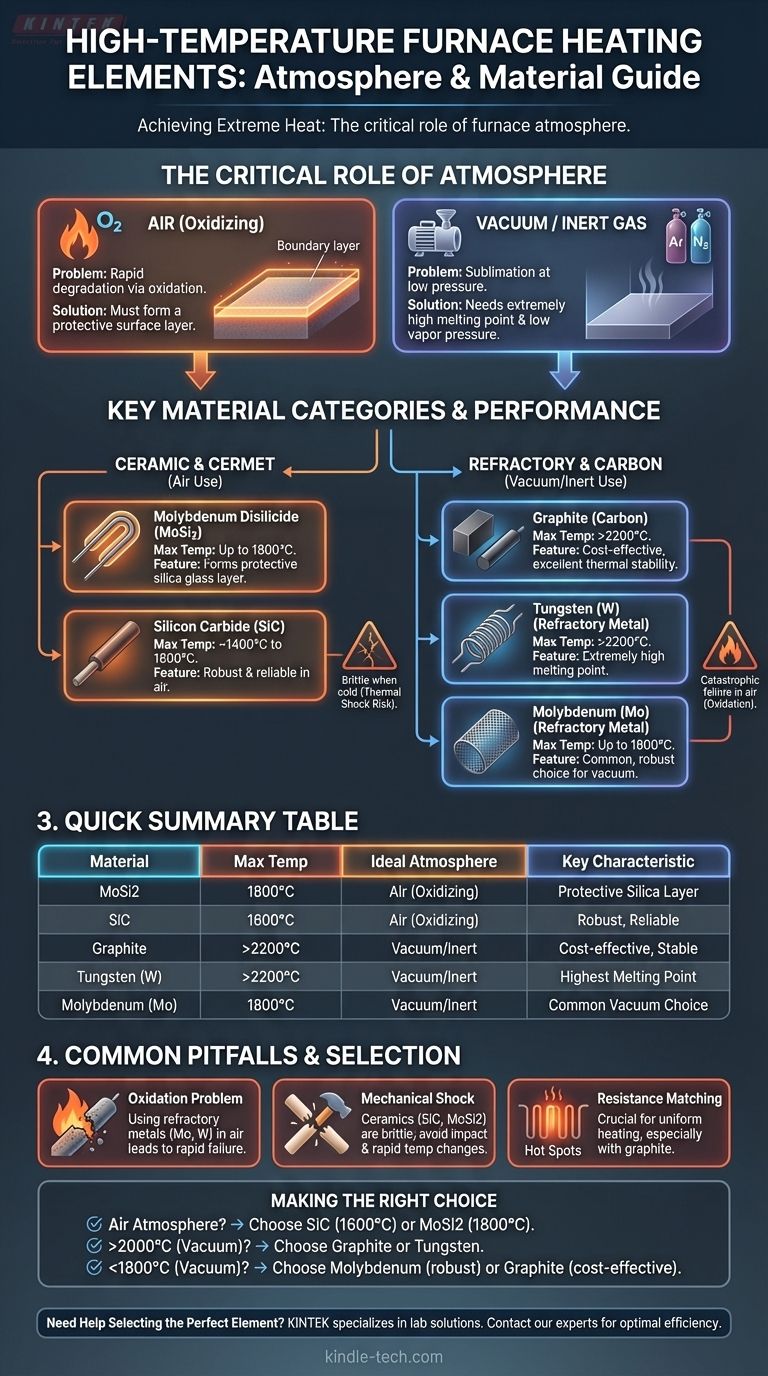
To achieve high temperatures, furnaces rely on specialized heating elements made from materials capable of withstanding extreme thermal stress without melting or degrading. The most common materials include molybdenum disilicide (MoSi2), silicon carbide (SiC), refractory metals like molybdenum and tungsten, and graphite, with the ideal choice depending heavily on the furnace's maximum temperature and internal atmosphere.
The selection of a high-temperature heating element is not just about a high melting point. It is a critical decision dictated by the furnace's operating atmosphere—whether it contains oxygen (air) or is a vacuum/inert environment—which determines which materials will survive.

The Critical Role of Furnace Atmosphere
The chemical environment inside the furnace at temperature is the single most important factor in choosing a heating element. Materials behave very differently in the presence of oxygen versus in a vacuum or inert gas.
Operation in Air
Elements that operate in an oxidizing atmosphere (air) must form a protective surface layer to prevent rapid degradation. This is their primary survival mechanism.
Operation in Vacuum or Inert Gas
In a vacuum or inert atmosphere, oxidation is not a concern. Here, the focus shifts to materials with extremely high melting points and low vapor pressure to prevent the element from sublimating (evaporating) at high temperatures.
Key Material Categories for High-Temperature Elements
Heating elements are typically grouped into a few key classes, each suited for different conditions and temperature ranges.
Ceramic and Cermet Elements
These materials are workhorses for high-temperature furnaces operating in air.
Molybdenum Disilicide (MoSi2) forms a protective silica glass layer when heated in air, allowing it to function at very high temperatures, often up to 1800°C. It is a cermet (ceramic-metal composite).
Silicon Carbide (SiC) is another ceramic element that performs well in air. It is robust and reliable for temperatures up to approximately 1400°C to 1600°C.
Refractory Metal Elements
These metals have exceptionally high melting points but will oxidize and fail almost instantly in air at high temperatures. They are strictly for vacuum or inert atmosphere furnaces.
Molybdenum (Mo) is a common choice for vacuum furnaces, suitable for temperatures up to around 1800°C.
Tungsten (W) has one of the highest melting points of any metal, allowing it to be used in vacuum furnaces reaching 2200°C or even higher.
Tantalum (Ta) is another refractory metal used in high-temperature vacuum applications, valued for its stability.
Carbon-Based Elements
Like refractory metals, carbon-based elements are used exclusively in vacuum or inert atmospheres.
Graphite is a cost-effective and very common heating element for vacuum furnaces. It has excellent thermal stability and can be used for temperatures exceeding 2200°C.
Precious Metal Elements
These are used in specialized applications where purity is paramount and cost is a secondary concern.
Platinum (Pt) and Rhodium (Rh) can operate in air at high temperatures and have high resistance to contamination. Rhodium’s very high melting point (1960°C) makes it suitable for demanding applications.
Common Pitfalls and Considerations
Choosing an element involves balancing performance, lifespan, and cost. Several factors can lead to premature failure.
The Oxidation Problem
Using a refractory metal like molybdenum or tungsten in an air atmosphere is the most common and catastrophic mistake. The element will rapidly burn out.
Mechanical Shock
Ceramic elements like SiC and MoSi2 are very brittle, especially when cold. They are susceptible to cracking from mechanical shock or rapid temperature changes.
Resistance Matching
For some furnace designs, particularly those with multiple graphite elements, it is crucial that the electrical resistance of the elements is closely matched to ensure uniform heating and prevent hot spots.
Making the Right Choice for Your Application
Your final decision must be aligned with your specific operational requirements.
- If your primary focus is operating in an air atmosphere: Silicon carbide (up to 1600°C) and Molybdenum Disilicide (up to 1800°C) are your best options.
- If your primary focus is reaching the highest possible temperatures (>2000°C): You must use a vacuum or inert gas furnace with graphite or tungsten elements.
- If your primary focus is a vacuum furnace below 1800°C: Molybdenum offers a robust and reliable solution, while graphite is often more cost-effective.
Ultimately, matching the heating element to the furnace atmosphere is the foundational principle for successful high-temperature work.
Summary Table:
| Material | Max Temperature | Ideal Atmosphere | Key Characteristics |
|---|---|---|---|
| Molybdenum Disilicide (MoSi2) | Up to 1800°C | Air (Oxidizing) | Forms protective silica layer; brittle when cold |
| Silicon Carbide (SiC) | Up to 1600°C | Air (Oxidizing) | Robust and reliable; susceptible to thermal shock |
| Graphite | > 2200°C | Vacuum/Inert | Cost-effective; excellent thermal stability |
| Tungsten (W) | > 2200°C | Vacuum/Inert | Extremely high melting point |
| Molybdenum (Mo) | Up to 1800°C | Vacuum/Inert | Common choice for vacuum furnaces |
Need Help Selecting the Perfect Heating Element?
Choosing the right heating element is critical for your furnace's performance and longevity. The wrong choice can lead to rapid failure and costly downtime.
KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our experts understand the intricate balance between temperature, atmosphere, and material properties. We can help you select the ideal element for your specific application, ensuring optimal efficiency and reliability.
Contact us today via the form below to discuss your high-temperature furnace requirements and get a personalized recommendation. Let KINTEK be your partner in achieving precise and reliable thermal processing.
Visual Guide
Related Products
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- Is molybdenum a good thermal conductor? Its High-Temperature Performance Explained
- What is the purpose of multi-stage electric heating in tensile testing? Achieve Precision in Grain Boundary Analysis
- What is the disadvantage of using tungsten? The High Cost and Difficulty of Fabrication
- Is tungsten the most heat resistant material? It depends on your application's environment.
- What is the process of resistance heating? A Guide to Efficient and Precise Thermal Control
- How are tubular heaters made? The Science Behind Durable & Efficient Heating Elements
- Why are high-performance sealing and refractory materials critical for high-temperature solar thermochemical reactors?
- What are the technical advantages of using graphite rods? Boost Precision in 1200°C High-Temperature Operations



















