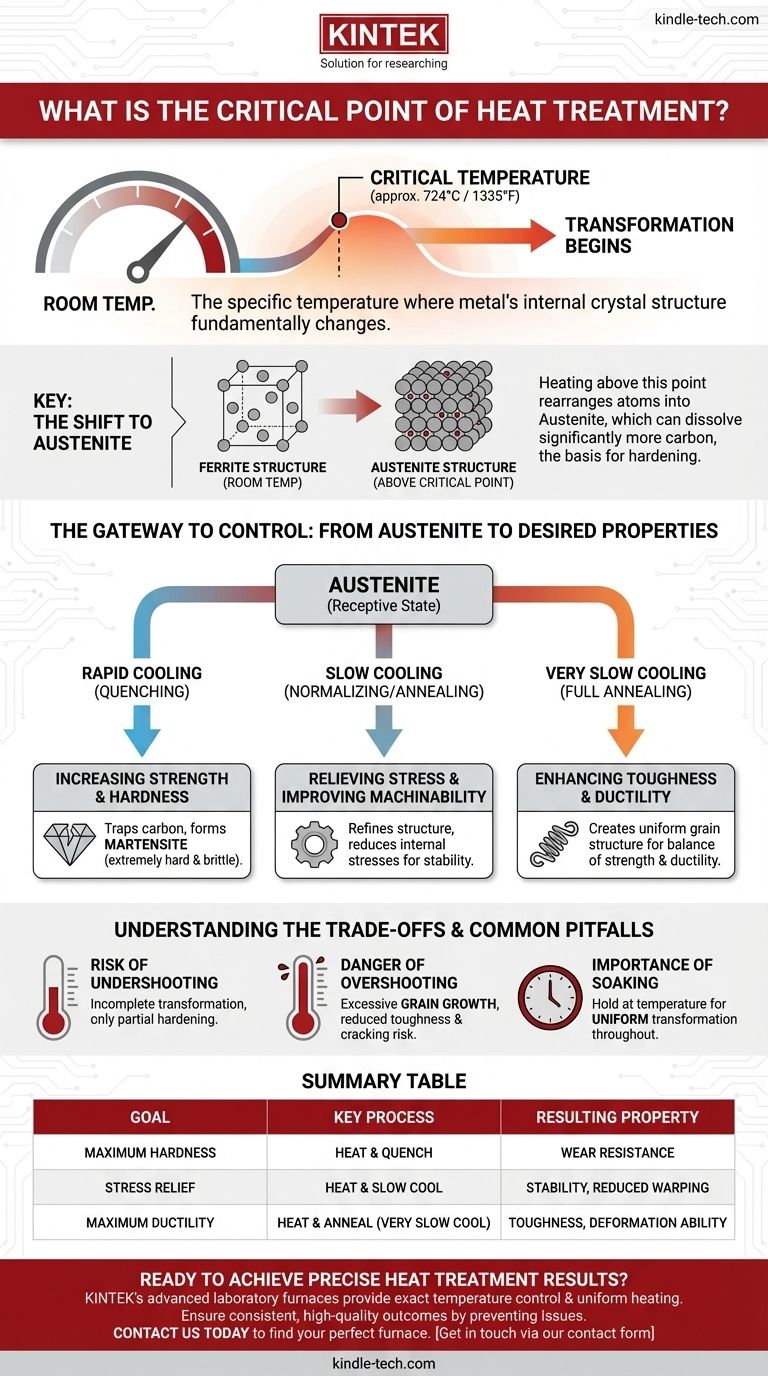
In heat treatment, the critical point refers to a specific temperature at which the internal crystal structure of a metal undergoes a fundamental change. For common steel, this transformation begins at approximately 724°C (1335°F). Heating above this temperature causes the steel's atoms to rearrange into a new structure called austenite, which is the essential first step for altering its mechanical properties.
Reaching the critical temperature isn't simply about making a metal hot; it's about unlocking its potential for transformation. Crossing this thermal threshold fundamentally restructures the material on an atomic level, making it receptive to being hardened, softened, or stabilized through controlled cooling.

The Mechanism: What Happens at the Critical Point?
The critical temperature is not an arbitrary number; it marks a precise phase transformation in the steel's crystalline lattice. Understanding this change is key to understanding all subsequent heat treatment processes.
The Shift to Austenite
At room temperature, steel exists in a crystal structure known as ferrite. When heated above its critical temperature, these crystals dissolve and recrystallize into a different, more compact atomic arrangement called austenite.
The Unique Role of Carbon
The new austenitic structure has a remarkable property: it can dissolve significantly more carbon than the room-temperature ferrite structure can. This ability to absorb carbon into the iron crystal lattice is the entire basis for hardening steel.
The Gateway to Control
Without first transforming the steel into austenite, processes like hardening and normalizing are impossible. Reaching the critical temperature is the non-negotiable first step that makes the steel's final properties controllable through subsequent cooling operations.
Practical Goals Achieved Through Critical Temperature
Heating a part past its critical temperature is done to achieve specific, tangible outcomes. The final properties are determined not just by reaching this temperature, but by how the steel is cooled from it.
Increasing Strength and Hardness
To make steel hard and wear-resistant, it is heated above the critical temperature until it becomes fully austenitic. It is then cooled very rapidly, a process called quenching. This rapid cooling traps the dissolved carbon atoms, creating a new, extremely hard and brittle structure called martensite.
Relieving Stress and Improving Machinability
After processes like welding or heavy forming, steel contains significant internal stresses. By heating it above the critical point and then cooling it slowly (a process called normalizing or annealing), the crystal structure can reform in a more uniform and stress-free state, making the part easier to machine and less prone to warping.
Enhancing Toughness and Ductility
While quenching makes steel very hard, it also makes it brittle. Other cooling rates from the austenitic state can be used to refine the grain structure, creating a final product that balances strength with ductility (the ability to deform without breaking).
Understanding the Trade-offs and Common Pitfalls
Precision is everything when dealing with critical temperatures. Both falling short and overshooting the target temperature range can compromise the integrity of the final part.
The Risk of Undershooting
Failing to reach the critical temperature means the transformation to austenite will be incomplete. If you then attempt to quench the part, only a small fraction of the material will harden, resulting in a failed heat treatment and a component that does not meet its design specifications.
The Danger of Overshooting
Heating the steel too far above the critical temperature, or holding it there for too long, causes the individual crystalline grains to grow excessively large. This condition, known as grain growth, can permanently reduce the steel's toughness and make it more susceptible to cracking.
The Importance of Soaking
Simply reaching the critical temperature is not enough. The component must be held at that temperature for a specific duration, known as soaking. This ensures the phase transformation to austenite occurs uniformly throughout the entire cross-section of the part, not just on the surface.
Making the Right Choice for Your Goal
Controlling the heat treatment process around the critical temperature allows you to tailor a material's properties to its intended application. Your goal dictates your method.
- If your primary focus is maximum hardness and wear resistance: You must heat the steel above its critical temperature to form austenite and then quench it rapidly.
- If your primary focus is relieving internal stress for stability: Heat the material past its critical point and allow it to cool slowly and uniformly, often in still air.
- If your primary focus is maximum softness and ductility: Use a full annealing process by heating above the critical temperature and then cooling it extremely slowly inside an insulated furnace.
Mastering the critical point is the key to unlocking and controlling the vast potential stored within a piece of steel.
Summary Table:
| Goal | Key Process | Resulting Property |
|---|---|---|
| Maximum Hardness | Heat above critical point, then quench | Wear resistance |
| Stress Relief & Machinability | Heat above critical point, then cool slowly | Stability, reduced warping |
| Maximum Ductility | Heat above critical point, then anneal (very slow cool) | Toughness, ability to deform |
Ready to achieve precise heat treatment results?
KINTEK's advanced laboratory furnaces provide the exact temperature control and uniform heating essential for reliably reaching and holding the critical point. Whether you are hardening tools, annealing components, or normalizing structures, our equipment ensures consistent, high-quality outcomes by preventing issues like incomplete transformation or grain growth.
Contact us today to find the perfect furnace for your specific steel transformation needs. Let KINTEK be your partner in precision. Get in touch via our contact form.
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the advantages of waste plastic pyrolysis? Transform Waste into Valuable Fuel and Chemicals
- What role does a two-stage rotary vane vacuum pump play in a radio frequency (rf) plasma carbonitriding system?
- What does sintering do? Transform Powder into Strong, Dense Components
- What is a resistance heating furnace? Achieve Precise, Clean High-Temperature Processing
- What are the disadvantages of vacuum evaporation? Understanding the Trade-offs in Thin-Film Deposition
- Why is a vacuum drying oven required for processing B4C-CeB6 ceramic precursor powders? Ensure Purity & Stability
- What is the difference between hardening and vacuum hardening? Choose the Right Process for Superior Surface Finish
- What are the important safety precautions for heat treatment? Protect Your Team from Extreme Heat and Invisible Hazards



















