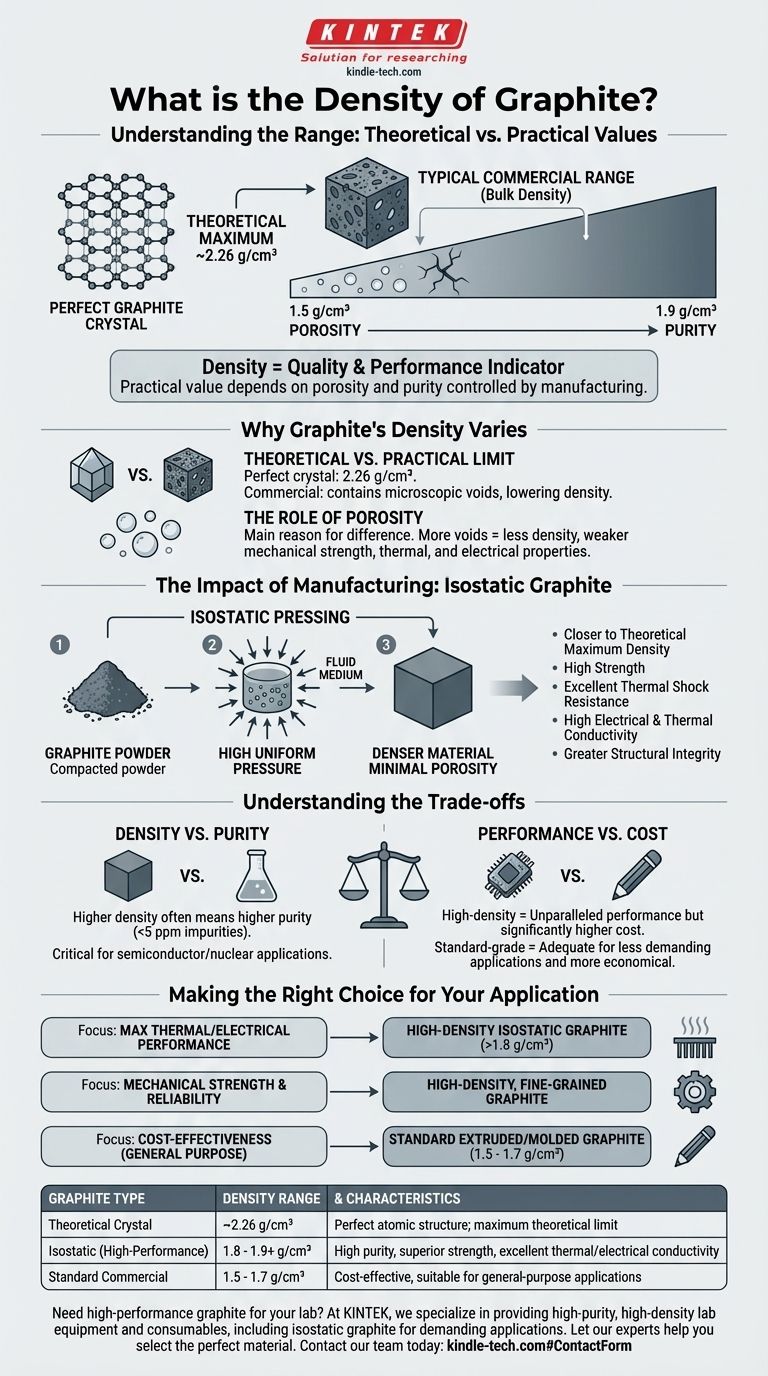
The density of graphite is not a single value but typically falls within a range, largely dependent on its form and manufacturing process. The theoretical maximum density of a perfect graphite crystal is approximately 2.26 g/cm³, but the bulk density of most manufactured graphite products ranges from 1.5 to 1.9 g/cm³. This variation is a direct result of the material's internal structure and purity.
The key takeaway is that graphite's density is a direct indicator of its quality and performance. While its theoretical crystal density is fixed, the practical density you encounter is determined by porosity and purity, which are controlled by the manufacturing method.

Why Graphite's Density Varies
Graphite's density is fundamentally tied to its atomic structure, but the real-world value is dictated by microscopic imperfections introduced during its formation or manufacturing.
The Theoretical vs. Practical Limit
A perfect graphite crystal consists of tightly packed layers of carbon atoms. The density derived from this ideal atomic arrangement is 2.26 g/cm³.
However, commercial graphite is never a perfect crystal. It contains microscopic voids or pores between its grains, which lowers the overall bulk density.
The Role of Porosity
Porosity is the primary reason for the difference between theoretical and bulk density. A less dense graphite block has more internal voids.
These voids can negatively impact mechanical strength, thermal conductivity, and chemical resistance by creating points of failure and interrupting the flow of heat or electricity.
The Impact of Manufacturing: Isostatic Graphite
To achieve properties suitable for demanding applications, synthetic graphite is engineered through specific manufacturing processes that minimize porosity. One of the most advanced methods is isostatic pressing, as mentioned in your references.
What Is Isostatic Pressing?
Isostatic pressing is a process where graphite powder is compacted using high pressure applied equally from all directions. This is typically done using a fluid medium.
This uniform pressure ensures that the resulting material has a highly consistent, fine-grained structure with minimal internal voids.
How Pressing Creates a Denser Material
By minimizing porosity, isostatic pressing creates a graphite product that is closer to its theoretical maximum density.
This higher density directly contributes to the superior properties noted in the references: high strength, excellent thermal shock resistance, and high electrical and thermal conductivity. A denser path allows energy to travel more efficiently and provides greater structural integrity.
Understanding the Trade-offs
Choosing a specific grade of graphite involves balancing performance requirements against practical constraints. The pursuit of maximum density is not always the most practical or cost-effective solution.
Density vs. Purity
Higher density often correlates with higher purity. Processes like isostatic pressing can produce graphite with impurity levels below 5 parts per million (ppm).
While this extreme purity is critical for semiconductor or nuclear applications, it comes at a significantly higher cost than standard-grade graphite.
Performance vs. Cost
A high-density, isostatically pressed graphite offers unparalleled performance in high-temperature and corrosive environments.
However, for less demanding applications like basic lubrication or pencils, a lower-density, more porous, and less expensive grade of graphite is perfectly adequate and more economical.
Making the Right Choice for Your Application
Your choice of graphite should be driven by the specific performance characteristics your project demands.
- If your primary focus is maximum thermal and electrical performance: Seek a high-density (above 1.8 g/cm³), isostatically pressed graphite for its low porosity and high purity.
- If your primary focus is mechanical strength and reliability: Choose a high-density, fine-grained graphite to minimize internal weak points and ensure resistance to thermal shock.
- If your primary focus is cost-effectiveness for a general-purpose application: A standard extruded or molded graphite with a lower density (1.5 - 1.7 g/cm³) will likely meet your needs.
Ultimately, understanding that density is a proxy for structural quality empowers you to select the precise material for your engineering goal.
Summary Table:
| Graphite Type | Typical Density Range (g/cm³) | Key Characteristics |
|---|---|---|
| Theoretical Crystal | ~2.26 | Perfect atomic structure; maximum theoretical limit |
| Isostatic (High-Performance) | 1.8 - 1.9+ | High purity, superior strength, excellent thermal/electrical conductivity |
| Standard Commercial | 1.5 - 1.7 | Cost-effective, suitable for general-purpose applications |
Need high-performance graphite for your lab? The density of your graphite directly impacts the success of your experiments and processes. At KINTEK, we specialize in providing high-purity, high-density lab equipment and consumables, including isostatic graphite for demanding applications. Let our experts help you select the perfect material to ensure superior thermal management, mechanical strength, and reliability.
Contact our team today to discuss your specific laboratory requirements and discover how KINTEK's solutions can enhance your results.
Visual Guide
Related Products
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the thermal limit of graphite? Unlock Extreme Heat Performance in Your Lab
- Why does graphite not melt? Unlocking the Secrets of Its Extreme Heat Resistance
- Why is graphite the best conductor of heat? Understanding Its Directional Thermal Superiority
- Is graphite affected by heat? Discover Its Remarkable Strength and Stability at High Temperatures
- What is the graphite furnace used for? Achieve Extreme Heat Up to 3000°C in a Controlled Environment



















