At its core, Chemical Vapor Deposition (CVD) is a material fabrication process that builds a solid, thin film on a surface from a chemical reaction in the gas phase. Precursor gases, containing the required atoms, are introduced into a reaction chamber where they are energized, typically by high heat. This energy causes the gases to react and decompose on or near a heated substrate, depositing a layer of the desired material atom-by-atom.
The essential mechanism of CVD is not merely condensation, but a controlled chemical transformation. It involves transporting gaseous reactants to a surface, using energy to trigger a specific chemical reaction that creates a solid, and then removing the gaseous byproducts, leaving behind a pure, engineered film.

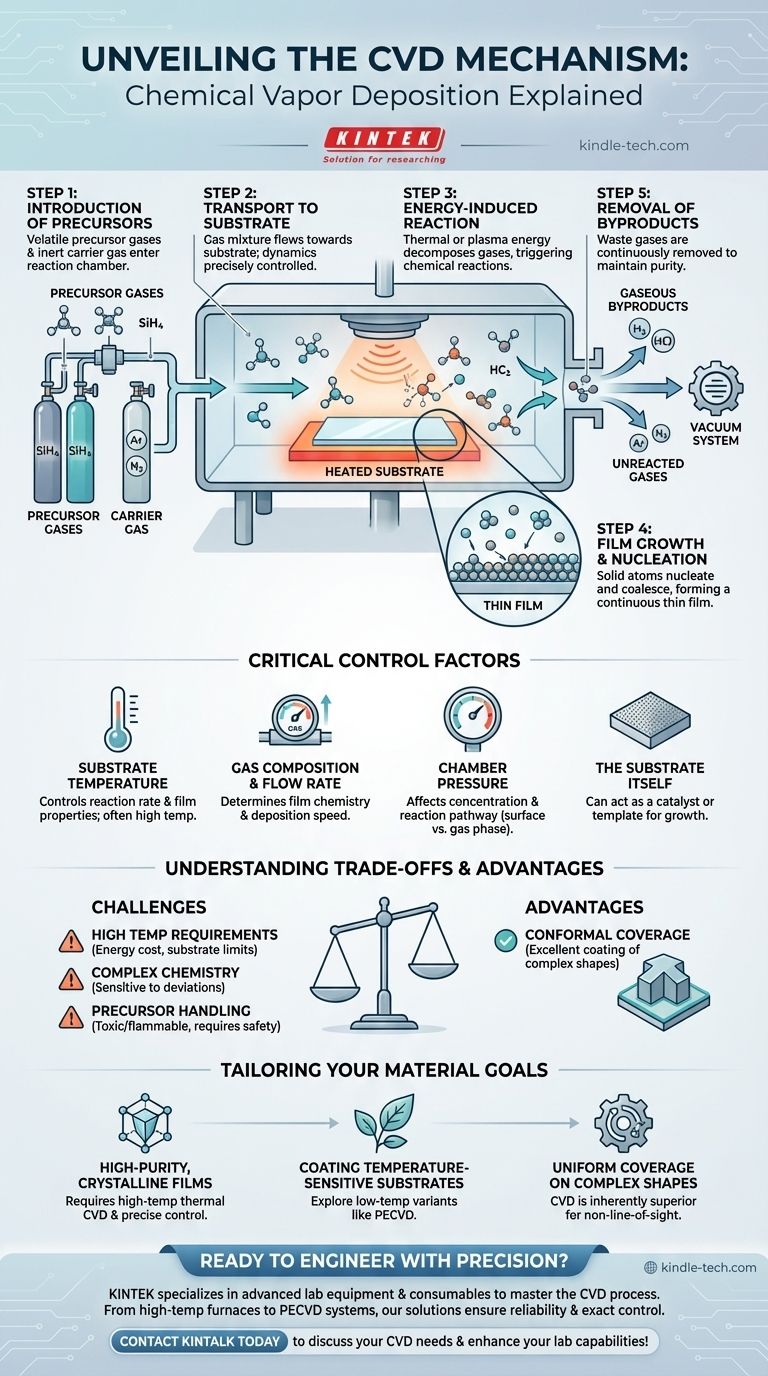
The Step-by-Step CVD Mechanism
To truly understand CVD, it's best to break it down into a sequence of distinct physical and chemical events that occur within the process chamber.
Step 1: Introduction of Precursors
The process begins by feeding one or more volatile precursor gases into a reaction chamber. These gases contain the molecular building blocks of the final film.
These reactive gases are often diluted with an inert carrier gas, such as argon or nitrogen, which helps control the reaction rate and ensure uniform delivery to the substrate.
Step 2: Transport to the Substrate
This mixture of gases flows through the chamber toward the substrate. The flow dynamics, pressure, and temperature within the chamber are all precisely controlled to ensure a stable and predictable delivery of reactants to the substrate surface.
Step 3: Energy-Induced Reaction
This is the heart of the CVD process. As the precursor gases get near or make contact with the heated substrate, they absorb thermal energy. In other variants, this energy can be supplied by a plasma.
This added energy is the catalyst that breaks chemical bonds in the precursor gases, causing them to decompose and react. This chemical reaction results in the formation of a solid material and gaseous byproducts.
Step 4: Film Growth and Nucleation
The solid atoms or molecules created by the reaction deposit onto the substrate surface. They begin to form stable clusters, or "nuclei," which then grow and coalesce to form a continuous, uniform thin film.
The substrate itself can act as a catalyst, providing a reactive surface that encourages the decomposition of the precursors and the adherence of the deposited film.
Step 5: Removal of Byproducts
The gaseous byproducts from the chemical reaction, along with any unreacted precursor and carrier gases, are removed from the chamber by a vacuum system. This continuous removal is critical for maintaining the purity of the film and driving the reaction forward.
The Critical Control Factors
The final properties of the deposited film—its thickness, purity, crystal structure, and uniformity—are dictated by several key process parameters.
Substrate Temperature
Temperature is arguably the most critical variable. It directly controls the rate of the chemical reactions on the surface. Too low, and the reaction won't occur; too high, and you might get undesirable phases or gas-phase reactions that lead to powder formation instead of a film.
Gas Composition and Flow Rate
The type of precursors used and their concentration in the carrier gas determine the chemistry of the final film. The flow rate dictates the supply of reactants to the surface, influencing the deposition speed.
Chamber Pressure
The pressure inside the chamber affects the concentration of gas molecules and their path to the substrate. It can influence whether reactions occur primarily on the surface (desired) or in the gas phase above it (undesired).
The Substrate Itself
The material and surface condition of the substrate can be a passive platform or an active participant. For example, in graphene growth, a copper substrate acts as a catalyst for the decomposition of carbon-containing gases and as a template for the graphene lattice to form.
Understanding the Trade-offs
While powerful, the CVD mechanism presents a distinct set of operational challenges and considerations that distinguish it from other methods like Physical Vapor Deposition (PVD).
High Temperature Requirements
Traditional thermal CVD often operates at very high temperatures (900–1400 °C). This high energy cost can limit the types of substrate materials that can be used without melting or degrading.
Complex Chemistry
The process relies on a delicate balance of chemical reactions. Small deviations in temperature, pressure, or gas purity can lead to different chemical pathways, resulting in impurities or incorrect film structure.
Precursor Handling
The precursor gases used in CVD can be highly toxic, flammable, or corrosive. This necessitates sophisticated safety protocols and handling equipment, which adds to the operational complexity and cost.
Conformal Coverage
A key advantage stemming from its gaseous nature is that CVD provides excellent conformal coverage. This means it can uniformly coat complex, three-dimensional shapes, which is a significant challenge for line-of-sight processes like PVD.
How This Applies to Your Material Goals
Understanding the CVD mechanism allows you to select and control the process to achieve specific outcomes for your material.
- If your primary focus is high-purity, crystalline films: You will need a high-temperature thermal CVD process with extremely precise control over gas purity and flow rates.
- If your primary focus is coating temperature-sensitive substrates (like polymers): You should explore low-temperature variants like Plasma-Enhanced CVD (PECVD), which uses RF plasma instead of high heat to energize the gas.
- If your primary focus is achieving uniform coverage on complex shapes: The fundamental gas-phase nature of CVD makes it an inherently superior choice over many line-of-sight deposition techniques.
Ultimately, understanding the CVD mechanism transforms it from a 'black box' process into a versatile and precise tool for engineering materials from the atom up.
Summary Table:
| CVD Step | Key Action | Outcome |
|---|---|---|
| 1. Introduction | Precursor gases enter the chamber | Building blocks for the film are supplied |
| 2. Transport | Gases flow to the heated substrate | Ensures uniform delivery of reactants |
| 3. Reaction | Energy decomposes gases on the substrate | Solid material and gaseous byproducts form |
| 4. Growth | Solid atoms nucleate and form a film | A continuous, uniform thin film is created |
| 5. Byproduct Removal | Gaseous waste is pumped away | Maintains film purity and drives the reaction |
Ready to Engineer Your Materials with Precision?
Understanding the CVD mechanism is the first step to achieving high-purity, uniform thin films for your research or production. KINTEK specializes in providing the advanced lab equipment and consumables you need to master this process.
Whether you require a high-temperature furnace for crystalline films or a Plasma-Enhanced CVD (PECVD) system for temperature-sensitive substrates, our solutions are designed for reliability and exact control. Let our experts help you select the perfect equipment to meet your specific material goals.
Contact KINTALK today to discuss your CVD needs and enhance your laboratory's capabilities!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Laboratory CVD Boron Doped Diamond Materials
People Also Ask
- What are the process capabilities of ICPCVD systems? Achieve Low-Damage Film Deposition at Ultra-Low Temperatures
- How do PECVD systems improve DLC coatings on implants? Superior Durability and Biocompatibility Explained
- Can plasma enhanced CVD deposit metals? Why PECVD is rarely used for metal deposition
- What is the process of PECVD in semiconductor? Enabling Low-Temperature Thin Film Deposition
- Why does a PECVD vacuum system require both a rotary vane and turbo pump? Ensure High-Purity Coatings



















