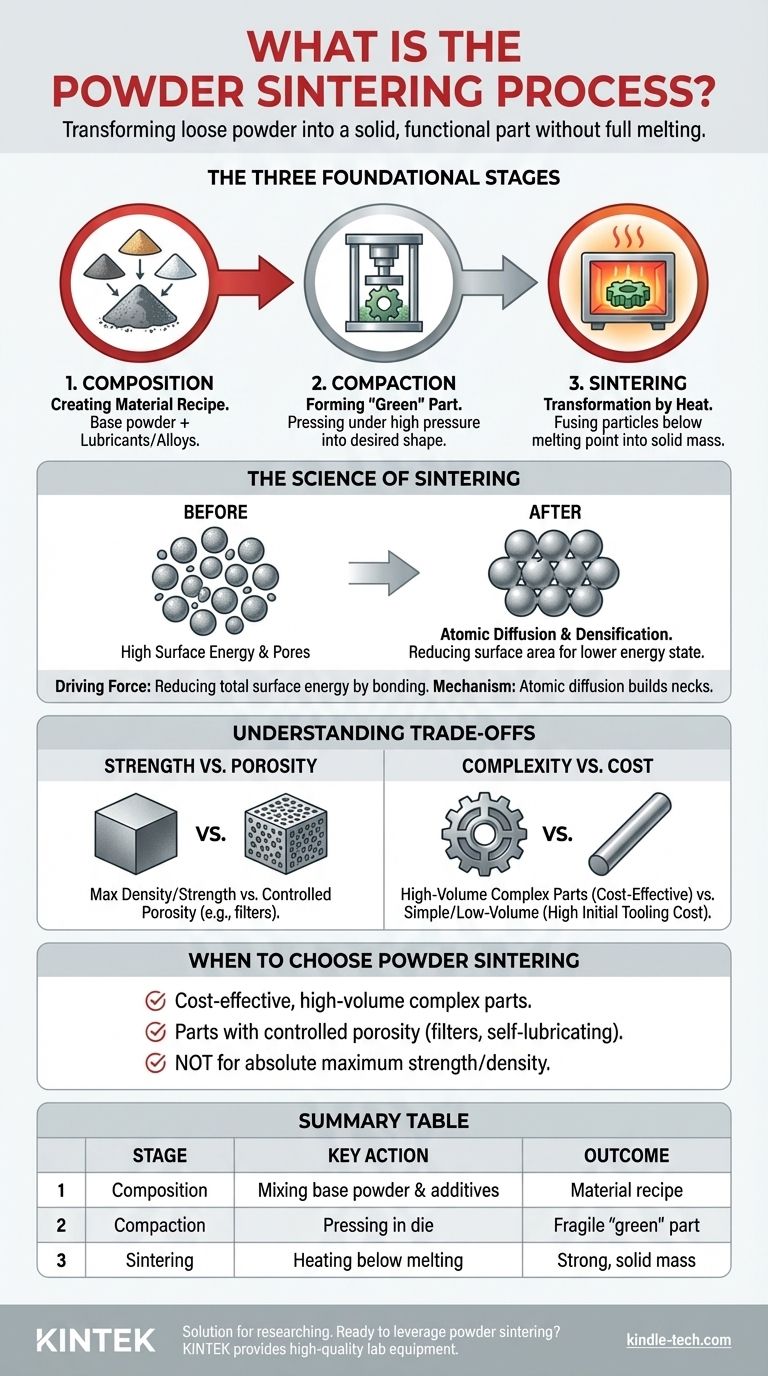
At its core, powder sintering is a manufacturing process that transforms loose powder into a solid, functional part without ever fully melting the material. It primarily involves three stages: first, a specific metal or ceramic powder composition is chosen and prepared; second, this powder is compacted under high pressure into a desired shape, known as a "green" part; and third, the green part is heated in a controlled furnace to a temperature just below its melting point, causing the individual powder particles to bond and fuse together.
Sintering is fundamentally a method of creating solid objects from powder by using heat and pressure to bond particles at an atomic level. Its main purpose is to produce complex, net-shape parts with high precision and minimal waste, often more cost-effectively than traditional machining or casting.

The Three Foundational Stages of Sintering
The sintering process is a highly controlled, sequential method. Each stage builds upon the last to transform raw powder into a finished component with specific mechanical properties.
Stage 1: Composition – Creating the Material Recipe
Before any shaping can occur, the raw material must be prepared. This involves selecting a primary metal (like iron, copper, or nickel) or ceramic powder that will determine the final part's core properties.
Often, other materials are added to this base powder. Lubricants are mixed in to improve the flow of powder into the die and reduce friction during compaction, while specific alloying elements can be added to enhance final strength, hardness, or corrosion resistance.
Stage 2: Compaction – Forming the "Green" Part
Once the powder mix is ready, it is fed into a die and subjected to immense pressure. This mechanical force presses the loose powder particles tightly together, forcing them into the desired shape.
This initial pressing creates what is known as a "green" part. The green part is solid enough to be handled but possesses only minimal strength, largely from the mechanical interlocking of particles and weak "cold welds" formed under pressure.
Stage 3: Sintering – The Transformation by Heat
The final and most critical stage is heating. The green part is placed in a furnace with a controlled atmosphere (to prevent oxidation) and heated to a high temperature, typically 70-90% of the material's absolute melting point.
The part is held at this temperature for a set period. This allows atomic-level processes to occur, which fuse the particles into a coherent, densified mass. After this, the part is cooled in a controlled manner to solidify its new, unified microstructure.
The Science of Sintering: How Heat Creates Strength
The sintering stage is not simply baking; it's a complex process of material science that fundamentally changes the part's internal structure. It works by exploiting the natural tendency of materials to exist in their lowest possible energy state.
The Driving Force: Reducing Surface Energy
Individual powder particles have a very high surface-area-to-volume ratio, which represents a high state of surface energy. Like water droplets that merge to form a larger, more stable drop, heated powder particles seek to reduce this energy.
By bonding together, the particles reduce their total exposed surface area, moving to a more stable, lower-energy state. This energy difference is the fundamental driving force behind the entire sintering process.
The Mechanism: Atomic Diffusion
This bonding doesn't happen through melting. Instead, at high temperatures, atoms become mobile and begin to diffuse across the boundaries where particles are touching. This migration of atoms builds "necks" or bridges between adjacent particles.
As these necks grow, the particles pull closer together. Several types of diffusion occur simultaneously—including surface, bulk, and grain boundary diffusion—all contributing to the formation of a solid, interconnected structure.
The Result: Densification and Pore Reduction
As atoms migrate and particles merge, the empty spaces (pores) between the original powder particles begin to shrink and close up. This leads to an increase in the part's overall density.
The final part is a single, unified mass with significantly improved strength, hardness, and other mechanical properties compared to the initial "green" compact.
Understanding the Trade-offs
While powerful, sintering is not the ideal solution for every application. Understanding its inherent trade-offs is key to making an informed engineering decision.
Strength vs. Porosity
The primary trade-off is between manufacturing ease and maximum density. Unless secondary operations are performed, most sintered parts retain some level of porosity. This makes them slightly less dense and strong than parts made by forging or machining from a solid billet.
However, this porosity can also be a key feature. It allows parts like bearings to be impregnated with oil for self-lubrication or enables the creation of filters with precisely controlled pore sizes.
Complexity vs. Cost
Sintering excels at producing small, highly complex parts in high volumes. Because parts are formed to their final or "net" shape, the need for costly secondary machining is drastically reduced or eliminated.
For simple shapes or low-volume production runs, the high initial cost of tooling (the die and press setup) may make other methods more economical.
When to Choose Powder Sintering
Your manufacturing choice should be dictated by your end goal. Sintering provides a unique set of capabilities that are ideal for certain applications.
- If your primary focus is cost-effective, high-volume production of complex parts: Sintering is an excellent choice, as it minimizes material waste and the need for post-process machining.
- If your primary focus is creating parts with controlled porosity (e.g., filters or self-lubricating bearings): Sintering is the definitive manufacturing method to achieve this specific goal.
- If your primary focus is achieving the absolute maximum material strength and density: You should consider alternative processes like forging, casting, or machining from a solid billet.
By understanding these core principles and trade-offs, you can confidently determine if sintering is the right engineering solution for your specific application.
Summary Table:
| Stage | Key Action | Outcome |
|---|---|---|
| 1. Composition | Mixing base powder with lubricants/alloys | Creates a material recipe for final properties |
| 2. Compaction | Pressing powder in a die under high pressure | Forms a fragile "green" part in the desired shape |
| 3. Sintering | Heating the green part below its melting point | Fuses particles into a strong, solid mass |
Ready to leverage powder sintering for your lab's needs? KINTEK specializes in providing the high-quality lab equipment and consumables essential for precise sintering processes. Whether you are developing new materials or manufacturing complex components, our expertise ensures you achieve consistent, high-quality results. Contact our specialists today to discuss how we can support your laboratory's sintering applications and help you optimize your manufacturing workflow.
Visual Guide
Related Products
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- Vacuum Dental Porcelain Sintering Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Spark Plasma Sintering Furnace SPS Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is one of the newest applications for dental ceramics? Monolithic Zirconia for Full-Arch Bridges
- What is the sintering temperature of zirconium? A Guide to the 1400°C-1600°C Range for Dental Labs
- What is the sintering time for zirconia? A Guide to Precise Firing for Optimal Results
- What is the price of zirconia sintering furnace? Invest in Precision, Not Just a Price Tag
- What is the temperature of sintering zirconia? Mastering the Protocol for Perfect Dental Restorations



















