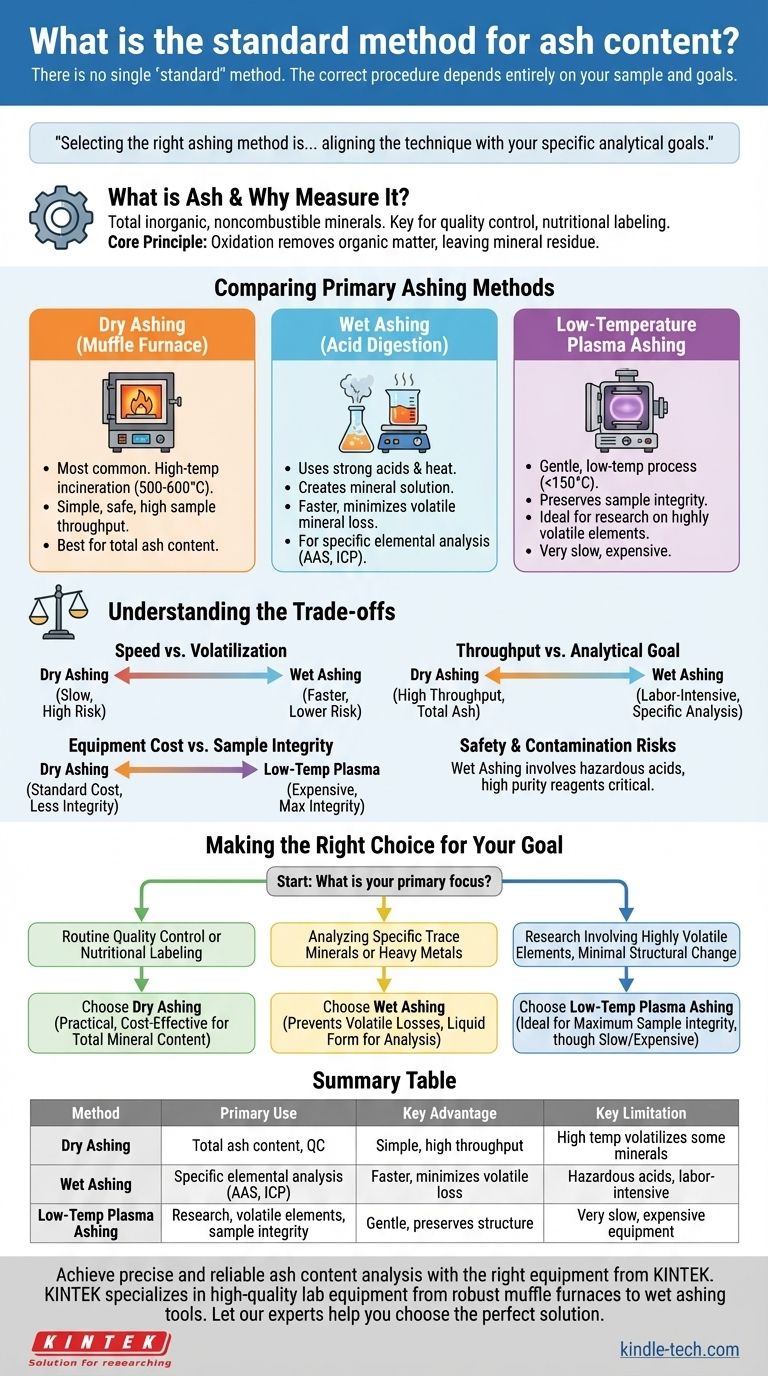
There is no single "standard" method for determining ash content because the correct procedure depends entirely on your sample and what you intend to measure. The most common and widely accepted approach for general purposes is dry ashing, but it is not universally applicable. The three primary methods—dry ashing, wet ashing, and low-temperature plasma ashing—each serve a distinct analytical purpose.
Selecting the right ashing method is less about finding a universal standard and more about aligning the technique with your specific analytical goals—whether you need a simple total mineral count or must preserve volatile elements for further, more detailed analysis.

What is Ash and Why Do We Measure It?
Ash content is a fundamental measure of the total amount of inorganic, noncombustible minerals within a sample. Understanding this is key to selecting the right method.
The Purpose of Ash Analysis
The primary goal of ashing is to remove all organic matter—compounds based on carbon, hydrogen, and nitrogen—leaving behind only the inorganic mineral residue. This residue is what we call "ash."
This measurement is critical in many fields, serving as a key indicator for quality control, nutritional labeling (total mineral content), and ensuring a product meets certain specifications.
From Sample to Ash: The Core Principle
All ashing methods operate on the same principle: oxidation. The process uses energy (heat or chemical) to break down the complex organic matrix into simple gases like carbon dioxide, water vapor, and nitrogen oxides, which then leave the sample.
The remaining material consists of the oxides, sulfates, phosphates, chlorides, and silicates of the inorganic elements present in the original sample, such as calcium, potassium, magnesium, and iron.
Comparing the Primary Ashing Methods
The choice between dry, wet, and low-temperature ashing comes down to a balance of speed, safety, cost, and the specific elements you need to analyze.
Dry Ashing (Muffle Furnace Method)
This is the most common method for determining total ash content. The sample is placed in a high-temperature muffle furnace, typically between 500°C and 600°C, and incinerated for several hours until only a white or gray ash remains.
It is simple, safe from a chemical handling perspective, and allows for many samples to be processed simultaneously with minimal supervision.
Wet Ashing (Acid Digestion)
This method uses strong acids (like nitric acid and sulfuric acid) and heat to chemically oxidize and dissolve the sample. It does not produce a dry ash but rather a mineral solution.
Wet ashing is primarily used when you need to analyze specific mineral elements after digestion using techniques like Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Plasma (ICP) analysis.
Low-Temperature Plasma Ashing
This is a highly specialized and much gentler technique. It uses a vacuum chamber where oxygen is excited into a plasma state. This reactive oxygen gas oxidizes the sample at much lower temperatures, typically below 150°C.
This method is ideal for research applications or when analyzing for extremely volatile minerals that would be lost even during wet ashing. However, the equipment is expensive and the process is very slow.
Understanding the Trade-offs
Each method comes with significant advantages and disadvantages. Objectively evaluating them is crucial for obtaining accurate data.
Speed vs. Volatilization
Dry ashing is very slow, often taking 8-12 hours or more. The extreme heat also poses a major risk of volatilization, where certain minerals (e.g., lead, zinc, mercury, iron) can be lost as vapor, leading to an inaccurate reading for those specific elements.
Wet ashing is much faster, often completed in under an hour. Because it operates at lower temperatures, it significantly reduces the loss of volatile minerals.
Sample Throughput vs. Analytical Goal
The simplicity of dry ashing makes it perfect for high-throughput quality control labs that need a total ash value for dozens of samples.
Wet ashing is more labor-intensive and requires constant attention, making it unsuitable for high sample numbers. Its purpose is not to measure total ash but to prepare a sample solution for precise elemental analysis.
Equipment Cost vs. Sample Integrity
Dry ashing requires only a standard muffle furnace, which is a common piece of lab equipment. Low-temperature plasma ashing, by contrast, requires expensive, specialized machinery.
The trade-off is sample integrity. For forensic analysis or research on the structure of the ash itself, the gentle nature of low-temperature ashing preserves the sample in a way no other method can.
Safety and Contamination Risks
Wet ashing involves handling highly corrosive and hazardous acids, requiring a fume hood and extensive personal protective equipment. Furthermore, the purity of the acids used is critical, as any mineral contaminants in the reagents will lead to falsely high results.
Making the Right Choice for Your Goal
Base your decision on your final analytical objective.
- If your primary focus is routine quality control or nutritional labeling: Dry ashing is the most practical and cost-effective method for determining total mineral content.
- If your primary focus is analyzing for specific trace minerals or heavy metals: Wet ashing is required to prevent volatile losses and prepare the sample in a liquid form for instrumental analysis.
- If your primary focus is research involving highly volatile elements with minimal structural change: Low-temperature plasma ashing is the ideal, albeit expensive and slow, method to ensure maximum sample integrity.
Ultimately, selecting the correct ashing method transforms a simple measurement into a precise and meaningful analytical result.
Summary Table:
| Method | Primary Use | Key Advantage | Key Limitation |
|---|---|---|---|
| Dry Ashing | Total ash content, quality control | Simple, high sample throughput | High temperature can volatilize some minerals |
| Wet Ashing | Specific elemental analysis (AAS, ICP) | Faster, minimizes volatile mineral loss | Requires hazardous acids, labor-intensive |
| Low-Temp Plasma Ashing | Research on volatile elements, sample integrity | Gentle process, preserves sample structure | Very slow, expensive equipment |
Achieve precise and reliable ash content analysis with the right equipment from KINTEK.
Selecting the correct ashing method is critical for accurate results in quality control, nutritional labeling, or advanced research. KINTEK specializes in providing the high-quality lab equipment you need—from robust muffle furnaces for dry ashing to the necessary tools for safe wet ashing procedures.
Let our experts help you choose the perfect solution for your laboratory's specific needs. Contact us today to discuss your application and enhance your analytical capabilities!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the hazards of a muffle furnace? Understanding the Critical Risks for Lab Safety
- What happens in the muffle furnace? Achieve Pure, Uniform High-Temperature Processing
- What is the construction of a muffle furnace? A Deep Dive into Its Core Systems
- What is the temperature range of a laboratory muffle furnace? Find the Right Model for Your Application
- How do you cool a muffle furnace? Safeguard your equipment and samples from thermal shock.



















