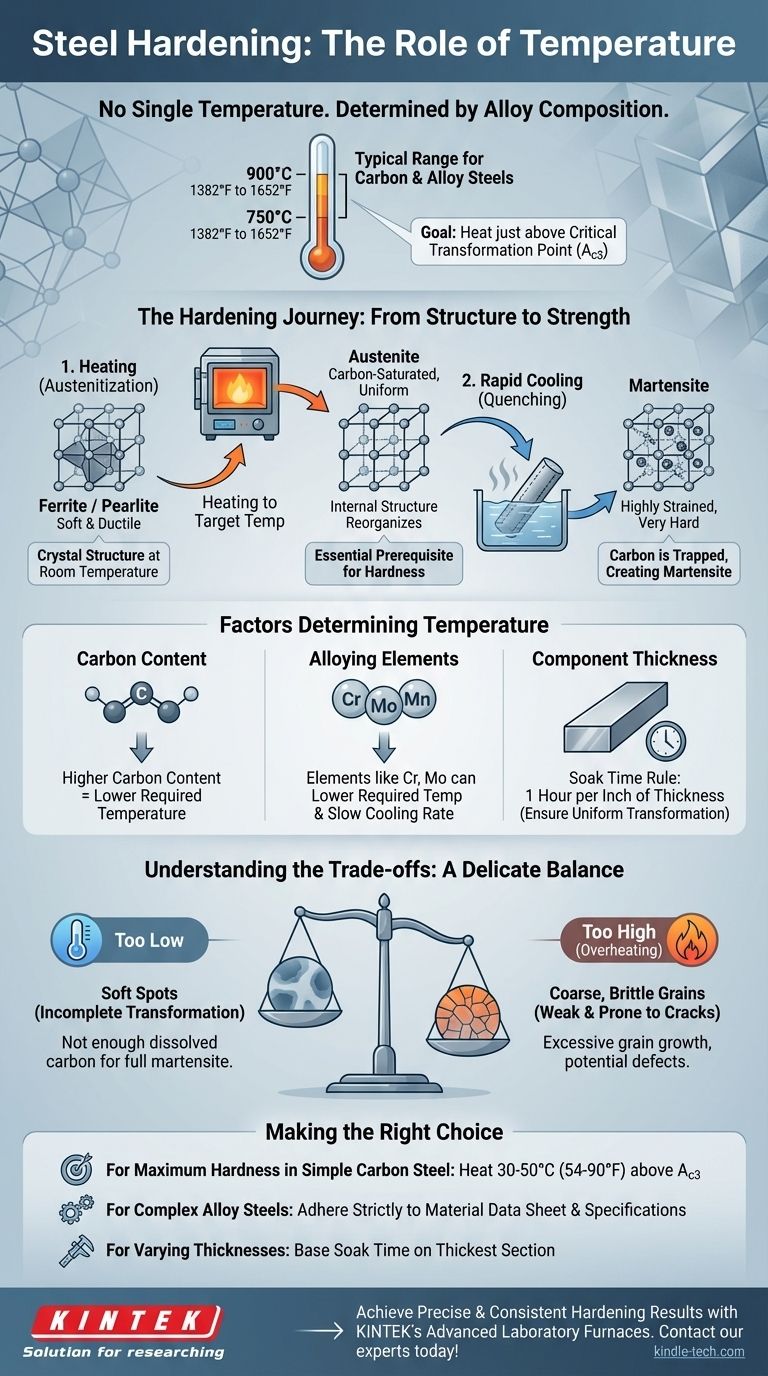
In steel hardening, there is no single temperature. The correct temperature is determined by the specific steel alloy's composition, aiming to heat it just above its critical transformation point—typically between 750°C and 900°C (1382°F to 1652°F) for most carbon and alloy steels—to change its internal crystal structure before rapidly cooling it.
The goal of heating is not to reach a universal "hardening temperature," but to heat a specific steel alloy just enough to transform its internal structure into austenite. This phase change is the essential prerequisite for achieving hardness upon cooling.

Why Temperature is a Target, Not a Rule
To understand hardening, we must look at the microscopic changes within the steel. The process is about deliberately manipulating the steel's crystal structure to create a harder, more wear-resistant state.
The Austenitic Transformation
Steel at room temperature has a crystal structure called ferrite or pearlite, which is relatively soft and ductile.
When you heat steel past its critical temperature (known as the A_c3_ point for many steels), this structure reorganizes into a new phase called austenite.
Austenite has a unique ability to dissolve carbon atoms within its crystal lattice. This is the fundamental goal of the heating stage: to create a uniform, carbon-saturated austenitic structure.
Locking in the Hardness
The "magic" of hardening happens during the rapid cooling, or quenching.
By cooling the steel quickly, the dissolved carbon atoms are trapped within the crystal structure. They don't have time to move back into their softer, room-temperature positions.
This trapped-carbon state creates a new, highly strained, and very hard structure known as martensite. It is the formation of martensite that gives hardened steel its characteristic properties.
Factors That Determine the Hardening Temperature
The precise temperature required is not arbitrary. It is dictated entirely by the steel's chemical makeup and the desired outcome.
Carbon Content
The amount of carbon in the steel is the single most important factor.
Steels with lower carbon content require higher temperatures to fully transform into austenite. Conversely, higher-carbon steels can transform at lower temperatures.
Alloying Elements
Elements like manganese, chromium, and molybdenum change the critical transformation temperatures.
These alloys can lower the required austenitizing temperature and, importantly, slow down the cooling rate needed to form martensite, making the hardening process more controllable.
Component Thickness
The material must be held at the target temperature long enough for the heat to penetrate evenly throughout its entire cross-section.
A common rule of thumb is to soak the material for one hour for every inch of thickness once it has reached the target temperature. This ensures a complete and uniform transformation to austenite.
Understanding the Trade-offs
Selecting the right temperature is a balancing act. Getting it wrong can ruin the material.
Heating Too Low
If the steel is not heated to a high enough temperature, the transformation to austenite will be incomplete.
This results in "soft spots" and a part that does not achieve its potential hardness, as there isn't enough dissolved carbon available to form a fully martensitic structure during the quench.
Heating Too High (Overheating)
Exceeding the optimal temperature can be even more damaging. It causes the grains within the steel's crystal structure to grow excessively large.
This leads to a coarse, brittle material that, while potentially hard, will be weak and prone to cracking under stress. In extreme cases, it can cause surface defects or even melting at the grain boundaries.
Making the Right Choice for Your Goal
The correct temperature is always specific to the material data sheet for the steel alloy you are working with. Always consult the manufacturer's or industry specifications.
- If your primary focus is maximum hardness in a simple carbon steel: Heat to approximately 30-50°C (54-90°F) above the steel's upper critical temperature (A_c3_) to ensure a full transformation.
- If you are working with a complex alloy steel: Adhere strictly to the recommended austenitizing temperature in the material's data sheet, as alloys significantly alter the transformation points.
- If your component has varying thicknesses: Base your soaking time on the thickest section of the part to ensure the core is fully transformed before quenching.
Ultimately, successful hardening depends on precise temperature control to achieve the foundational austenitic structure necessary for creating strength.
Summary Table:
| Factor | Influence on Hardening Temperature |
|---|---|
| Carbon Content | Higher carbon = lower temperature; Lower carbon = higher temperature. |
| Alloying Elements | Elements like Cr, Mo can lower the required temperature. |
| Component Thickness | Thicker sections require longer soak times at temperature. |
| Goal | Maximum hardness vs. controlled hardening for complex alloys. |
Achieve precise and consistent hardening results with KINTEK's advanced laboratory furnaces.
Our equipment delivers the exact temperature control and uniform heating essential for transforming steel into austenite, the critical first step to creating a durable, martensitic structure. Whether you're working with carbon steels or complex alloys, KINTEK's solutions ensure you avoid the risks of soft spots or brittleness from incorrect temperatures.
Ready to optimize your heat treatment process? Contact our experts today to find the perfect furnace for your specific steel and application requirements.
Visual Guide
Related Products
- Vertical Laboratory Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- How do you clean a tubular furnace tube? A Step-by-Step Guide to Safe and Effective Maintenance
- What temperature is tube annealing? A Guide to Material-Specific Ranges for Optimal Results
- What is the temperature of a quartz tube furnace? Master the Limits for Safe, High-Temp Operation
- What is the process of annealing tubes? Achieve Optimal Softness and Ductility for Your Tubing
- What is the difference between upflow and horizontal furnace? Find the Perfect Fit for Your Home's Layout



















