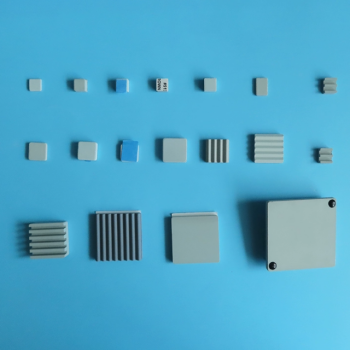
In an air environment, the thermal stability of single-layer graphene (SLG) begins to degrade at approximately 500°C. At this temperature, defects start to appear in the material's structure, compromising its integrity.
Graphene's thermal stability is not a single, fixed value. It is fundamentally determined by two factors: the surrounding environment (specifically the presence of oxygen) and its structural form (the number of layers).

The Critical Role of Environment and Structure
Understanding what influences graphene's heat tolerance is crucial for any practical application. The ideal properties of a perfect graphene sheet differ from its real-world performance under thermal stress.
The Impact of Oxygen
The primary mechanism for graphene's thermal degradation in air is oxidation. Oxygen molecules in the atmosphere react with the carbon atoms, especially at elevated temperatures.
This reaction effectively "burns" the graphene sheet, creating structural defects like holes and vacancies that destroy its unique electronic and mechanical properties.
Single-Layer vs. Bilayer Graphene
The number of layers has a direct and significant impact on thermal stability. Each additional layer provides a protective effect for the layers underneath.
According to studies, single-layer graphene (SLG) starts showing defects around 500°C. In contrast, bilayer graphene (BLG) is much more robust, remaining stable until about 600°C.
The Graphene-to-Graphite Comparison
To put this in context, it's useful to compare graphene to its bulk form, graphite. The tightly packed, multi-layered structure of graphite offers superior protection against oxidation.
As a result, bulk graphite remains intact even at 700°C in air, demonstrating a clear trend: more layers lead to higher thermal stability.
Understanding the Trade-offs
The theoretical stability of graphene can be misleading without considering the realities of its physical form and production.
The Vulnerability of Defects
Real-world graphene sheets are not perfectly uniform. They contain defects, grain boundaries, and edges that serve as initial points of attack for oxidation.
These reactive sites are where the degradation process begins, meaning a higher-quality, more pristine graphene sheet will generally exhibit better thermal performance.
Production Method Matters
The method used to synthesize the graphene, such as Chemical Vapor Deposition (CVD), influences its quality.
Factors like the catalyst used and growth conditions determine the final material's defect density. This means that the thermal stability can vary between graphene samples produced by different methods or even different labs.
How to Apply This to Your Project
Your application's specific operating conditions will determine the type of graphene you need.
- If your primary focus is an application in air below 500°C: Single-layer graphene is a suitable choice, but be mindful of potential long-term degradation near this temperature limit.
- If your primary focus is stability in air above 500°C: You must consider using bilayer or few-layer graphene for its superior resistance to oxidation.
- If your primary focus is a high-temperature application in a vacuum or inert gas: The stability of single-layer graphene is significantly higher, as the primary degradation mechanism (oxidation) has been removed.
Successfully leveraging graphene requires matching the material's environmental and structural limitations to your specific operational goals.
Summary Table:
| Graphene Type | Thermal Stability in Air (Approx.) | Key Factor |
|---|---|---|
| Single-Layer Graphene (SLG) | 500°C | Most vulnerable to oxidation |
| Bilayer Graphene (BLG) | 600°C | Additional layer provides protection |
| Bulk Graphite | 700°C | Multi-layered structure offers highest stability |
Need high-quality graphene or expert advice for your high-temperature application?
At KINTEK, we specialize in providing advanced lab equipment and materials, including high-purity graphene suited for demanding environments. Whether you're working with single-layer, bilayer, or custom graphene solutions, our team can help you select the right material to ensure stability and performance in your specific conditions.
Contact us today to discuss your project requirements and discover how KINTEK's solutions can enhance your research and development.
Visual Guide
Related Products
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Square Lab Press Mold for Laboratory Applications
- Cylindrical Lab Electric Heating Press Mold for Laboratory Applications
- Square Bidirectional Pressure Mold for Lab Use
People Also Ask
- What are the applications of graphite material? Leveraging Extreme Heat and Precision for Industrial Processes
- Why graphite is used in furnace? Achieve Superior Heat Treatment & Energy Efficiency
- Does graphite have a melting point? Unlocking the Extreme Heat Resistance of Graphite
- What is the temperature of a graphite furnace? Achieve Extreme Heat Up to 3000°C
- Why is graphite used in furnaces? For Extreme Heat, Purity, and Efficiency