Fundamentally, FTIR spectroscopy can analyze nearly any type of sample, including solids, liquids, and gases. The critical factor is not the sample's initial state, but how it is prepared to be sufficiently transparent to infrared (IR) light for analysis. The chosen preparation method is what allows the instrument to obtain a clear and interpretable spectrum.
The core challenge of FTIR sampling isn't if your material can be analyzed, but how to prepare it. Your choice of technique—from pressing a solid into a pellet to placing a liquid between salt plates—directly depends on the sample's physical state and your analytical goals.

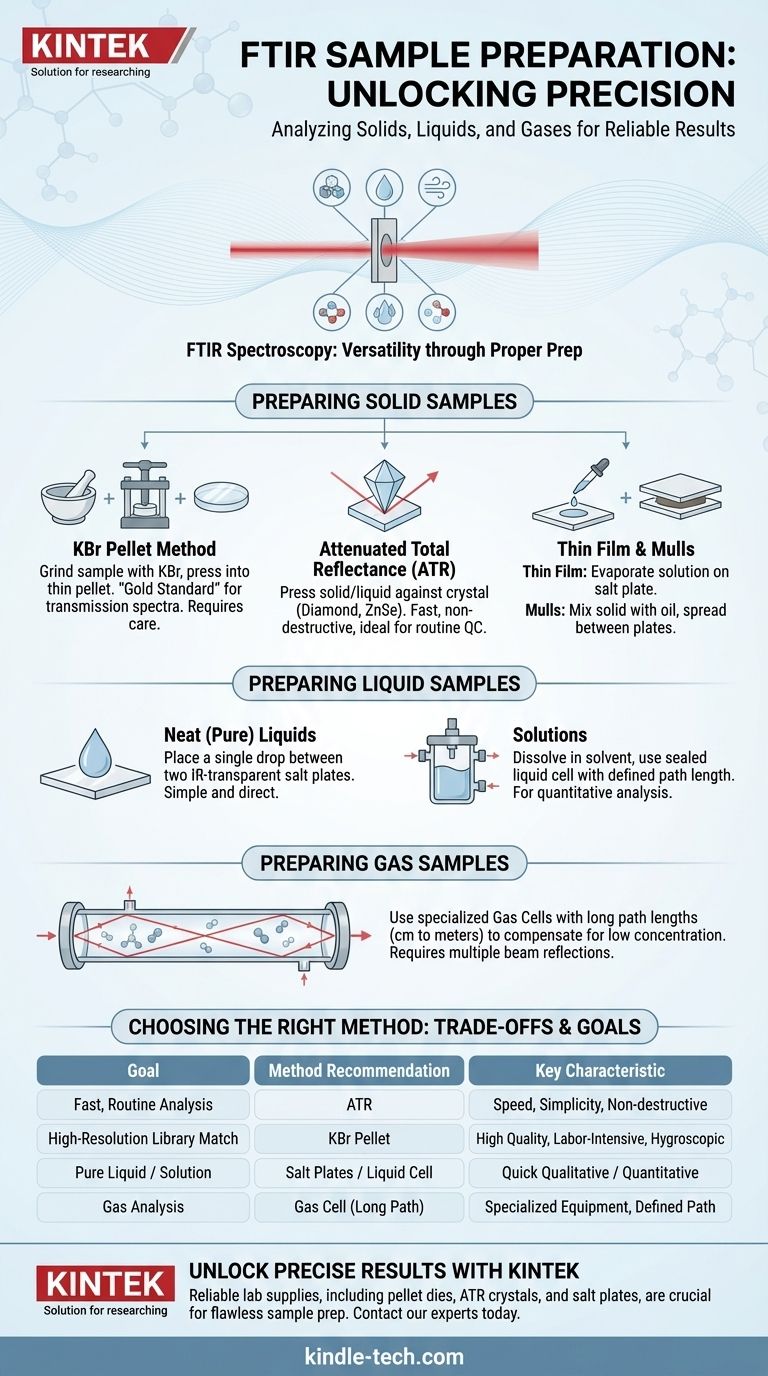
Preparing Solid Samples
Solid analysis offers the most variety in preparation techniques. The goal is always to make the sample thin enough or disperse it enough for the IR beam to pass through or interact with it effectively.
The KBr Pellet Method
This is a classic transmission technique. A very small amount of your solid sample (typically 1-2 mg) is finely ground with a larger amount of spectroscopic-grade potassium bromide (KBr) powder.
This mixture is then compressed under high pressure using a pellet die and press to form a small, thin, semi-transparent disc. KBr is used because it is transparent to IR radiation and has a plastic-like quality under pressure that allows it to form a stable matrix for the sample.
Attenuated Total Reflectance (ATR)
ATR is arguably the most common and convenient method used today. It requires minimal or no sample preparation.
The solid (or liquid) is simply pressed into direct contact with a high-refractive-index crystal, typically made of diamond, zinc selenide, or germanium. The IR beam reflects internally within the crystal, creating an "evanescent wave" that penetrates a few micrometers into the sample surface, generating a spectrum.
Thin Film Analysis
If your solid can be dissolved in a volatile solvent, you can prepare a thin film.
A drop of the solution is placed on an IR-transparent salt plate (like KBr or NaCl). The solvent is then gently evaporated, leaving behind a thin, uniform film of the solid ready for direct analysis.
Mulls
In this technique, the solid is ground into a fine paste with a mulling agent, most commonly Nujol (a mineral oil).
This paste is then spread thinly between two IR-transparent salt plates. The main drawback is that the spectrum of the mulling agent itself will be superimposed on your sample's spectrum, which can obscure important regions.
Preparing Liquid Samples
Analyzing liquids is generally more straightforward than solids, as they can easily form the thin layers required for analysis.
Neat (Pure) Liquids
The simplest method involves placing a single drop of a pure liquid between two salt plates. The plates are gently squeezed together to form a very thin capillary film. This "sandwich" is then placed directly in the spectrometer's sample holder.
Solutions
If the sample is a solid that needs to be dissolved, or if a pure liquid absorbs too strongly, it can be analyzed as a solution.
The sample is dissolved in a solvent that has minimal IR absorption in the region of interest (e.g., carbon tetrachloride or chloroform). This solution is then injected into a sealed liquid cell with a precisely defined path length for quantitative analysis.
Preparing Gas Samples
Gas analysis requires specialized equipment due to the very low concentration of molecules compared to liquids and solids.
Gas Cells
Gases are analyzed using a gas cell, which is a tube with IR-transparent windows at both ends.
To get a strong enough signal, these cells are designed to have a long path length, often using internal mirrors to reflect the beam back and forth through the gas multiple times. Path lengths can range from a few centimeters to many meters.
Understanding the Trade-offs
Each sampling method has distinct advantages and disadvantages. Choosing the right one is critical for obtaining reliable data.
Ease of Use vs. Spectral Quality
ATR is the champion of speed and simplicity, making it ideal for routine quality control. However, the resulting spectrum can sometimes differ subtly from a classic transmission spectrum due to variations in penetration depth.
The KBr pellet method, while labor-intensive and highly sensitive to moisture contamination, is capable of producing exceptionally high-quality transmission spectra that are often considered the "gold standard" for library matching.
Sample Integrity and Contamination
The KBr pellet and mull techniques are destructive; the sample is mixed with another material and cannot be easily recovered. ATR is non-destructive, a major advantage when dealing with precious samples.
Mulls introduce spectral interference from the mulling agent. KBr pellets are hygroscopic (they absorb water from the air), meaning a water peak is a very common artifact if the KBr powder is not kept perfectly dry.
Choosing the Right Method for Your Sample
Your choice should be guided by your sample's physical form and what you need to learn from the analysis.
- If your primary focus is fast, routine analysis of a solid or non-volatile liquid: Use ATR for its unmatched speed and minimal sample prep.
- If your primary focus is obtaining a high-resolution library-quality spectrum of a solid: Use the KBr pellet method, but be prepared for the meticulous preparation required.
- If your primary focus is analyzing a pure liquid or a sample in solution: Use a pair of salt plates for a quick qualitative scan or a sealed liquid cell for quantitative work.
- If your primary focus is analyzing a gas or a mixture of gases: A dedicated gas cell with an appropriate path length is the only effective option.
By matching the preparation technique to your sample's state and analytical goal, you can unlock precise and reliable results from your FTIR analysis.
Summary Table:
| Sample Type | Common Preparation Methods | Key Characteristics |
|---|---|---|
| Solids | KBr Pellet, ATR, Thin Film, Mull | Versatile; method choice impacts spectral quality and ease of use. ATR is fast and non-destructive. |
| Liquids | Neat (Salt Plates), Solution Cell | Simple preparation; forms thin layers easily. Ideal for qualitative and quantitative analysis. |
| Gases | Gas Cell (Long Path Length) | Requires specialized cell; long path length compensates for low molecule concentration. |
Unlock precise FTIR results with the right equipment from KINTEK. Whether you're preparing solid KBr pellets, analyzing liquids with salt plates, or working with gases, having reliable lab supplies is crucial. KINTEK specializes in high-quality FTIR consumables and accessories, including pellet dies, presses, ATR crystals, and salt plates, designed to meet the diverse needs of laboratory professionals. Ensure your sample prep is flawless—contact our experts today to find the perfect solution for your FTIR analysis!
Visual Guide
Related Products
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
- Electrolytic Electrochemical Cell for Coating Evaluation
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Customizable Swagelok Type Test Cells for Advanced Battery Research Electrochemical Analysis
People Also Ask
- Are hydraulic presses dangerous? Understanding the Risks and Safety Measures for Your Lab
- What are the weakness of hydraulic press? Understand the Trade-offs of Power and Control
- How is a hydraulic press utilized in the assembly of all-solid-state battery anodes? Optimize Indium Foil Interfaces
- What are two problems that could arise in the preparation of a KBr pellet for IR analysis? Avoid Moisture & Grinding Errors
- What can XRF identify? Discover the Elements in Your Materials with Precision
- What are the advantages of pressed pellet technique? Enhance Precision & Accuracy in Sample Analysis
- What is the importance of using a laboratory hydraulic press for R1/3Zr2(PO4)3 samples? Enhance Ion Conductivity
- Why is high-precision pressure control equipment required for the production of lead-antimony alloy components? Accuracy for Longevity



















