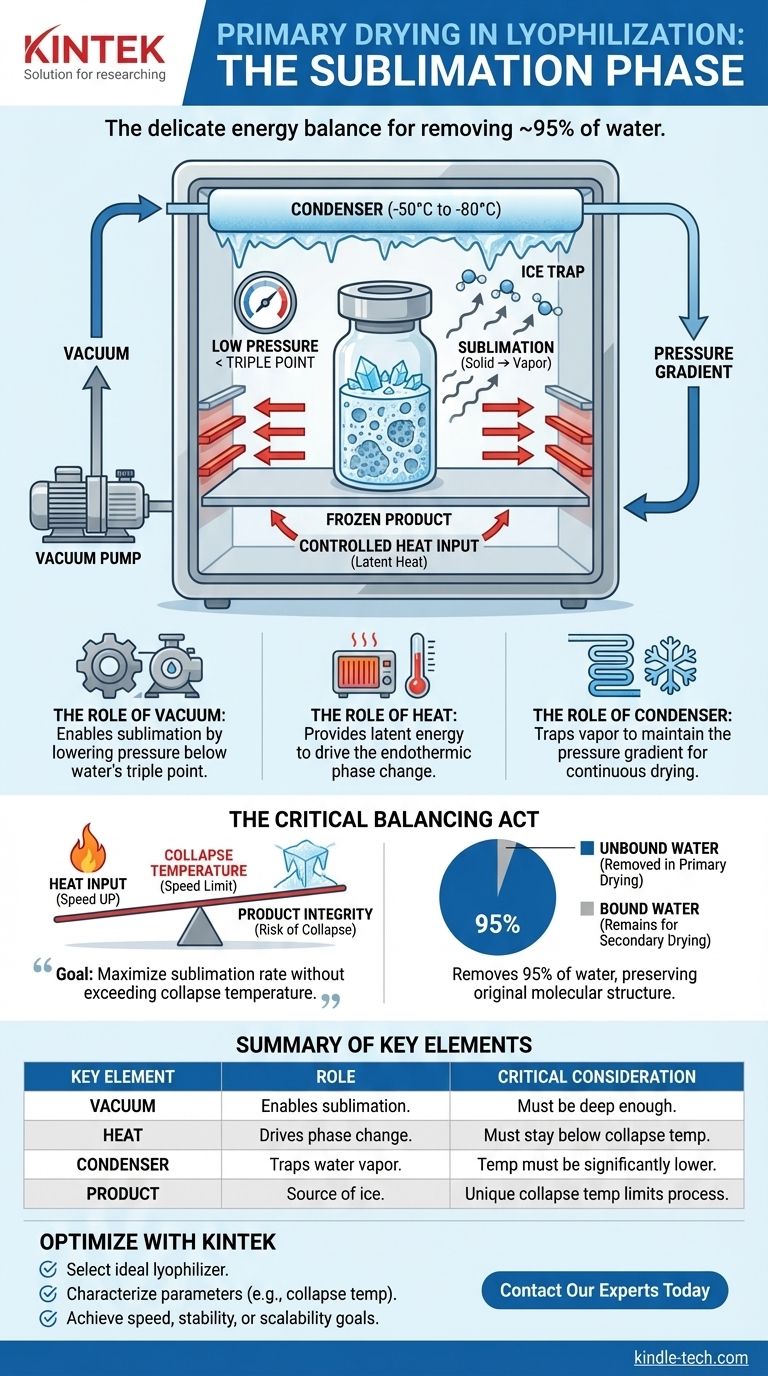
During the primary drying phase of lyophilization, the core process is sublimation, where frozen water in the material is converted directly into a vapor without passing through a liquid phase. This is achieved by lowering the chamber pressure to a deep vacuum and carefully adding a controlled amount of heat to the product. The vacuum pump and a cold condenser work together to draw this water vapor away, effectively removing up to 95% of the water from the product.
Primary drying is not about aggressive heating, but about a delicate energy balance. The goal is to provide just enough thermal energy to drive sublimation while keeping the product's temperature below its critical structural failure point, known as the collapse temperature.

The Core Mechanics of Sublimation
The primary drying phase is the longest and most critical stage in the entire lyophilization cycle. It sets the foundation for the final product's stability and structure. This process relies on the precise interplay of pressure, temperature, and heat transfer.
Creating the Environment: The Role of Vacuum
Lowering the pressure within the lyophilizer chamber is the first and most crucial step. This deep vacuum reduces the pressure well below the triple point of water (6.11 mbar, 0.01°C).
At this low pressure, water can no longer exist as a liquid. This environment forces the solid ice to transition directly into a gas (water vapor) when energy is applied, a process known as sublimation.
Driving the Process: The Role of Heat
Sublimation is an endothermic process, meaning it requires energy to occur. Heat is carefully introduced, typically by warming the shelves the product vials are sitting on.
This added energy is the latent heat of sublimation, which gives the ice molecules the energy they need to escape into the vapor phase. Without this controlled heat input, the process would be incredibly slow, as sublimation would cause the product to cool, eventually stopping the process altogether.
Capturing the Result: The Role of the Condenser
As water vapor leaves the product, it must be removed from the chamber to maintain the low-pressure environment. This is the job of the condenser.
The condenser is a surface within the lyophilizer that is held at an extremely low temperature (often -50°C to -80°C). The water vapor migrates from the warmer product to the colder condenser, where it freezes back into ice. This effectively traps the water, maintains the pressure gradient, and continuously drives the sublimation process forward.
Understanding the Critical Trade-offs
While the principles are straightforward, successful primary drying is a balancing act. Pushing the process too quickly can irrevocently damage the product.
The Balancing Act: Heat vs. Product Integrity
The primary challenge is to remove water as quickly as possible without causing product collapse. Adding more heat speeds up sublimation, but it also increases the product's temperature.
If the heat input is too aggressive, the product temperature can rise to a critical point where its structure softens and is no longer able to support itself.
The Collapse Temperature: The Ultimate Speed Limit
This critical point is known as the collapse temperature. For crystalline products, this is the eutectic melting temperature. For amorphous products (like many biologics), it is the glass transition temperature (Tg).
Exceeding this temperature, even slightly, causes the rigid, porous structure to melt and collapse. This results in a loss of the desired cake structure, difficulty in rehydration, and often a complete loss of biological activity for sensitive pharmaceuticals.
Why This Phase Removes 95% of the Water
This phase is responsible for removing all the unbound or "free" water that was frozen into ice crystals. This represents the vast majority of water in the product, typically around 95%.
The slow, meticulous nature of this phase is precisely why it is so effective at preserving the product's original molecular structure, as the rigid ice matrix acts as a scaffold until it is fully sublimated away. The remaining ~5% of water is "bound" to the product molecules and is removed in the next stage, secondary drying.
Optimizing Primary Drying for Your Goal
The ideal parameters for primary drying depend entirely on the nature of your product and your operational goals. Understanding your priorities is key to developing a robust and efficient cycle.
- If your primary focus is process speed: Your goal is to run the process as close to the collapse temperature as possible without exceeding it, maximizing the sublimation rate.
- If your primary focus is product stability (e.g., biologics): Prioritize keeping the product temperature well below the collapse temperature, even if it significantly lengthens the drying time.
- If your primary focus is developing a new cycle: Invest in product characterization (e.g., using a freeze-drying microscope) to accurately determine the collapse temperature before you begin process optimization.
Mastering this delicate energy transfer is the absolute key to creating a stable, elegant, and effective lyophilized product.
Summary Table:
| Key Element | Role in Primary Drying | Critical Consideration |
|---|---|---|
| Vacuum | Lowers chamber pressure below water's triple point, enabling sublimation. | Must be sufficiently deep to prevent liquid phase. |
| Heat | Provides the latent heat of sublimation to drive the phase change from ice to vapor. | Must be controlled to stay below the product's collapse temperature. |
| Condenser | Traps water vapor by freezing it, maintaining the pressure gradient for continuous drying. | Temperature must be significantly lower than the product temperature. |
| Product | The frozen material from which ice sublimates, leaving a porous structure. | Its unique collapse temperature is the ultimate limit for the process. |
Optimize Your Lyophilization Process with KINTEK
Mastering the delicate balance of primary drying is essential for producing stable, high-quality lyophilized products. Whether you are developing a new cycle for a sensitive biologic or aiming to increase the efficiency of an existing process, having the right equipment and expertise is critical.
KINTEK specializes in advanced laboratory equipment and consumables for all your lyophilization needs. We can help you:
- Select the ideal lyophilizer with precise control over temperature and pressure.
- Characterize your product's critical parameters, like collapse temperature, for robust cycle development.
- Achieve your goals for speed, product stability, or process scalability.
Ready to enhance your freeze-drying outcomes? Contact our experts today via our contact form to discuss how we can support your laboratory's success.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Low-Temperature Water-Cooled Touchscreen Vibratory Ultrafine Pulverizer
- Desktop Fast High Pressure Laboratory Autoclave Sterilizer 16L 24L for Lab Use
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Laboratory Vibratory Sieve Shaker Machine Slap Vibrating Sieve
People Also Ask
- In which fields is the laboratory freeze dryer commonly used? Essential for Biopharma, Food Science & Research
- What is the recommended approach to selecting features for a lab freeze dryer? Match Core Performance to Your Application
- What are the overall benefits of freeze drying technology across industries? Achieve Unparalleled Product Preservation
- How does freeze drying benefit the cosmetics industry? Unlock Potent, Preservative-Free Formulas
- What is the basic process of freeze drying? A Guide to Lyophilization Stages and Benefits



















