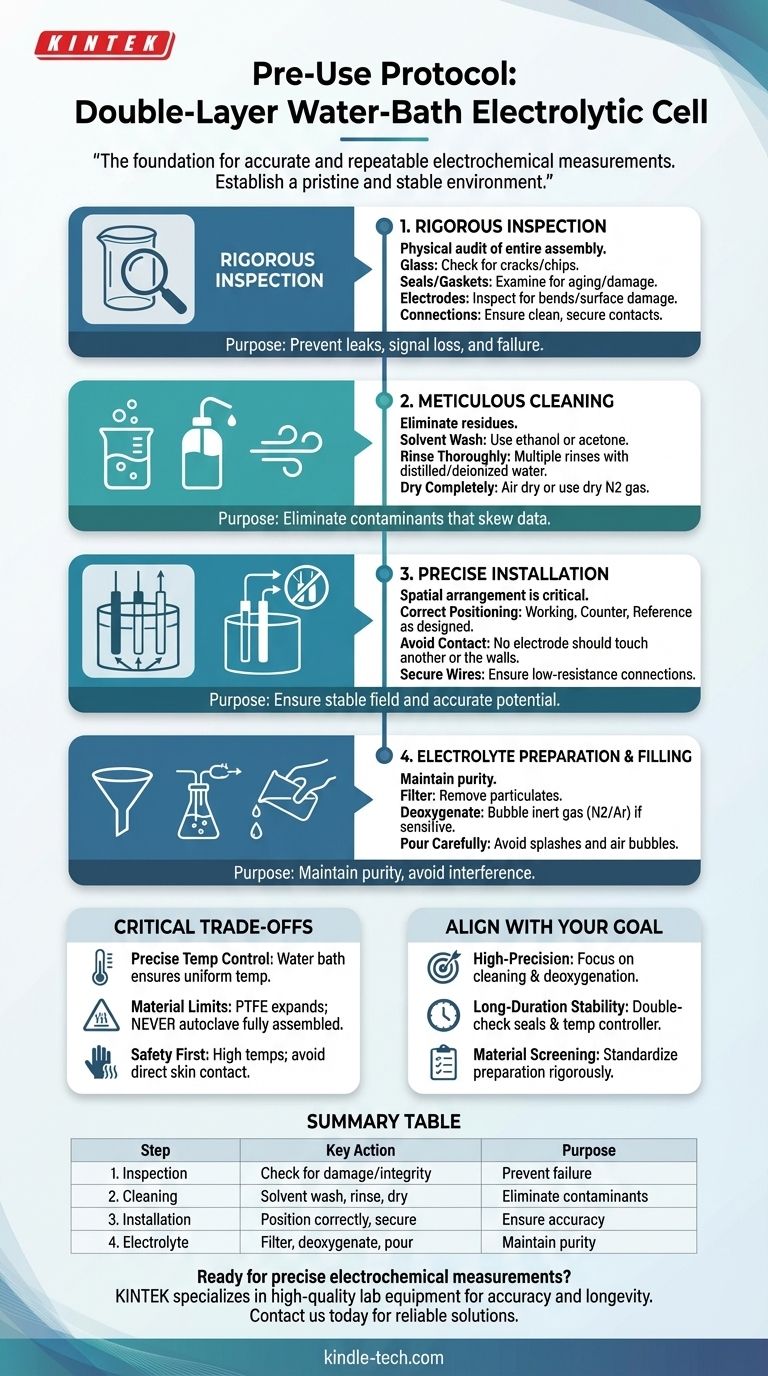
Before any experiment begins, the reliability of your results is determined by your preparation. For a double-layer water-bath electrolytic cell, there are four essential pre-use steps: a thorough inspection for damage, meticulous cleaning to remove contaminants, precise installation of electrodes, and careful preparation and loading of the electrolyte. Following this sequence is not merely a formality; it is the foundation for accurate and repeatable electrochemical measurements.
The core purpose of pre-use procedures is to establish a pristine and stable environment for your reaction. Every step, from inspecting glass for microcracks to deoxygenating your electrolyte, is designed to eliminate variables that could compromise the integrity of your data.

The Four Pillars of Pre-Experiment Preparation
A systematic approach to setup is non-negotiable. Each of the following steps addresses a potential source of experimental error, ensuring that the data you collect reflects your reaction, not your setup.
Step 1: Rigorous Inspection
Before introducing any chemicals, perform a physical audit of the entire cell assembly. Your goal is to identify any pre-existing faults that could cause leaks, signal loss, or total failure.
- Glass Cell Body: Check carefully for any cracks or chips. Even a small fracture can become a critical failure point under thermal stress from the water bath.
- Seals and Gaskets: Examine all seals, such as the PTFE lid, for signs of aging, brittleness, or damage. Compromised seals can lead to electrolyte leakage or failure to maintain an inert atmosphere.
- Electrodes: Inspect the working, reference, and auxiliary electrodes. Look for any bends, physical deformities, or surface damage. The electrode surface is where the reaction occurs, and its integrity is paramount.
- Connections: Ensure all gas tubes, salt bridge tubes, and electrical contacts are clean and can be connected securely. A loose connection will create noise and instability in your measurements.
Step 2: Meticulous Cleaning
Electrochemical measurements are extremely sensitive to impurities. Residues from previous experiments or storage can act as unintended reactants or catalysts, skewing your results.
- Solvent Wash: Begin by cleaning the cell body with a suitable solvent, such as ethanol or acetone, to remove organic grease and residues.
- Rinse Thoroughly: After the solvent wash, rinse the cell multiple times with distilled or deionized water to remove the solvent and any inorganic salts.
- Dry Completely: Ensure the cell is perfectly dry before assembly. You can air dry it or, for faster results, use a stream of dry nitrogen gas. This prevents unintended dilution of your electrolyte.
Step zIndex 3: Precise Installation
The spatial arrangement of the electrodes in a three-electrode system directly influences the electrochemical field and, therefore, the results.
- Correct Positioning: Install the working, counter (auxiliary), and reference electrodes as specified by your experimental design.
- Avoid Contact: Critically, ensure that no electrode is touching another electrode or the walls of the cell. Such contact can cause a short circuit and will invalidate the experiment.
- Secure Wires: Confirm that the wires connecting the electrodes to your potentiostat have a good, low-resistance connection. Poor contact leads to unstable signals and inaccurate potential control.
Step 4: Electrolyte Preparation and Filling
The electrolyte is the medium for your reaction. Its purity and handling are just as important as the cell itself.
- Filter if Necessary: If your prepared electrolyte contains any visible particulates, filter it before use.
- Deoxygenate the Solution: If your reaction is sensitive to oxygen, you must deoxygenate the electrolyte by bubbling an inert gas (like nitrogen or argon) through it. Oxygen is electrochemically active and can produce a significant interfering signal.
- Pour Carefully: Pour the electrolyte slowly into the cell, avoiding splashes. Introducing air bubbles can be problematic, as bubbles adhering to an electrode surface block the active area and interfere with measurements.
Understanding the Critical Trade-offs
A double-layer cell offers superior temperature control, but this feature introduces its own set of constraints and potential pitfalls.
The Purpose of the Water Bath
The outer jacket is not just for insulation; it is an active control system. By circulating water from a constant-temperature bath, you ensure the internal cell temperature is precise and uniform. This is vital because reaction rates, diffusion coefficients, and electrode kinetics are all highly dependent on temperature.
Thermal Expansion and Material Limits
The reference materials warn that the PTFE lid will expand when heated and may not return to its original shape. For this reason, you must never autoclave the fully assembled cell. While the glass components can be sterilized at high temperatures, the plastic or PTFE parts cannot.
Safety is Paramount
The water bath can operate at high temperatures. Always be cautious and avoid direct skin contact with the water bath apparatus or the electrolytic cell itself during operation to prevent burns.
Making the Right Choice for Your Goal
Your experimental objective should guide the level of rigor you apply to each step.
- If your primary focus is high-precision quantitative analysis: Pay extreme attention to the cleaning and electrolyte deoxygenation steps, as even trace contaminants or dissolved oxygen can alter your results.
- If your primary focus is long-duration stability testing: Double-check all seals and ensure the water bath temperature controller is stable and reliable for the entire duration of the experiment.
- If your primary focus is screening new materials: Standardize your preparation protocol rigorously. Every cell must be inspected, cleaned, and assembled identically to ensure your comparisons are valid.
Ultimately, disciplined and consistent preparation is the foundation of trustworthy electrochemical research.
Summary Table:
| Step | Key Action | Purpose |
|---|---|---|
| 1. Inspection | Check for cracks, damaged seals, and electrode integrity | Prevent leaks, signal loss, and experimental failure |
| 2. Cleaning | Wash with solvent (e.g., ethanol), rinse with distilled water, and dry | Eliminate contaminants that skew electrochemical data |
| 3. Electrode Installation | Position electrodes correctly, avoid contact, secure wires | Ensure stable electrochemical field and accurate measurements |
| 4. Electrolyte Preparation | Filter, deoxygenate with inert gas, and pour carefully | Maintain purity and avoid interference from oxygen or particulates |
Ready to achieve precise and reliable electrochemical measurements?
Proper setup is critical for success. KINTEK specializes in high-quality lab equipment and consumables, including durable electrolytic cells and accessories designed for accuracy and longevity. Our products help laboratories minimize experimental error and enhance reproducibility.
Contact us today to discuss your specific needs and discover how KINTEK can support your research with reliable solutions.
Get in touch with our experts now!
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
People Also Ask
- What are the primary applications of the all-quartz electrolytic cell? Essential for High-Purity & Optical Analysis
- Why is a quartz electrolytic cell used for acrylic acid wastewater? Ensure Chemical Stability & Data Integrity
- How should an all-quartz electrolytic cell and its components be maintained for long-term use? A Guide to Maximizing Equipment Lifespan
- What are the standard opening specifications for sealed and unsealed all-quartz electrolytic cells? Optimize Your Electrochemistry Setup
- What is the proper procedure for post-experiment cleanup and storage of an all-quartz electrolytic cell? Ensure Longevity and Reproducibility



















