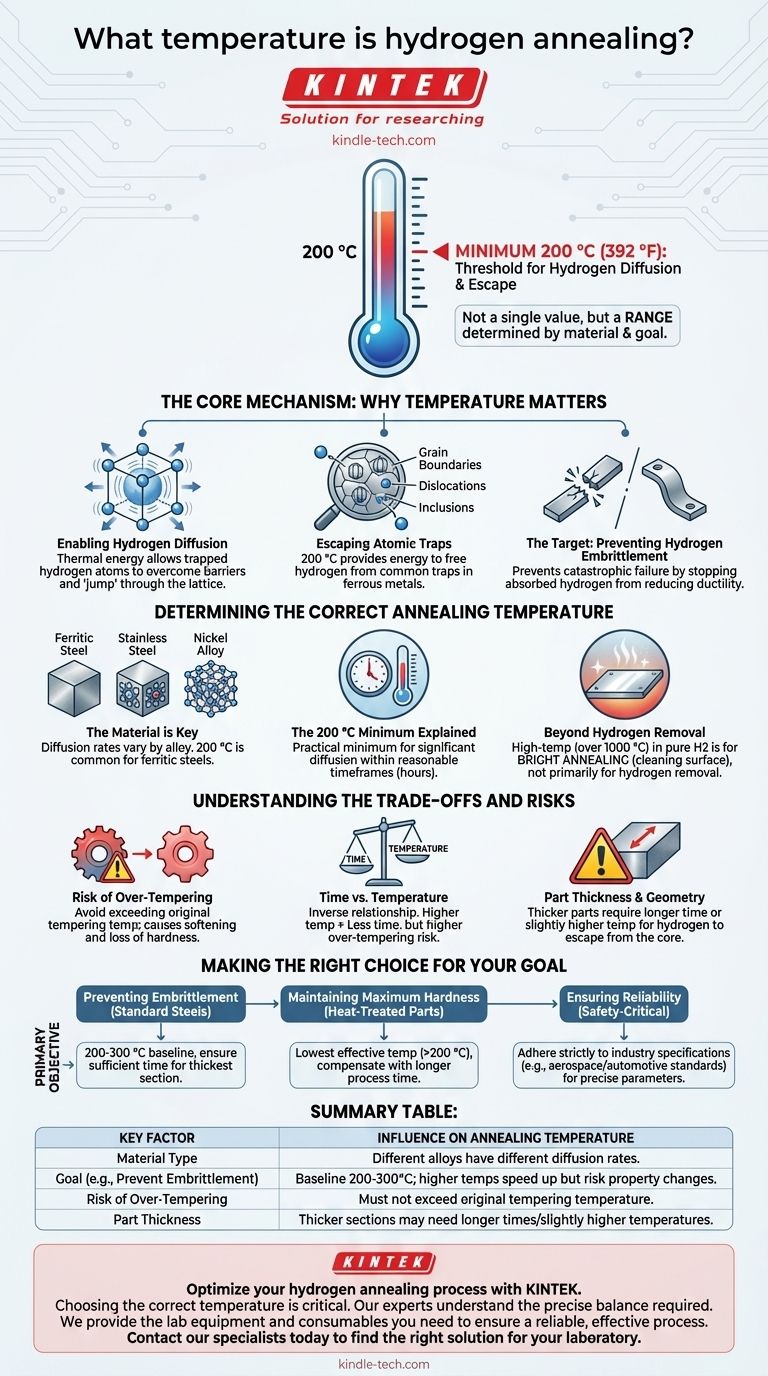
In hydrogen annealing, the process temperature is not a single value but a range determined by the material and the specific goal, with a minimum of 200 °C often cited for hydrogen removal. This temperature is the threshold at which hydrogen atoms gain enough thermal energy to diffuse out of the crystal lattice of materials like iron and steel, which is the primary mechanism for preventing hydrogen embrittlement. The actual temperature selected balances the speed of hydrogen removal against potential negative effects on the material's properties.
The goal of hydrogen annealing is not simply to heat a material, but to provide just enough energy for trapped hydrogen to escape. The correct temperature is a critical balance: high enough to enable this atomic diffusion, but low enough to avoid damaging the component's desired mechanical properties, such as hardness.

The Core Mechanism: Why Temperature Matters
Hydrogen annealing, often called a "hydrogen bake-out," is a dehydrogenation process. Its effectiveness is fundamentally tied to temperature's role in atomic mobility.
Enabling Hydrogen Diffusion
Temperature is a measure of thermal energy. For hydrogen atoms trapped within a metal's structure, this energy allows them to overcome the barriers that hold them in place. Below a certain temperature, the hydrogen is effectively locked in, but as the temperature rises, the atoms vibrate more intensely and can "jump" from one position in the lattice to another, eventually reaching the surface and escaping.
Escaping Atomic Traps
Hydrogen atoms don't just sit freely inside a metal. They are attracted to and trapped at defects in the crystal structure, such as grain boundaries, dislocations, and inclusions. The 200 °C minimum represents the energy level needed for hydrogen to begin escaping these common traps in ferrous metals.
The Target: Preventing Hydrogen Embrittlement
The ultimate purpose of this process is to prevent hydrogen embrittlement. This is a catastrophic failure mechanism where absorbed hydrogen significantly reduces a material's ductility and fracture toughness. A component that would normally bend under load may instead fracture suddenly and without warning, making hydrogen removal a critical step for safety-critical parts.
Determining the Correct Annealing Temperature
While 200 °C is a common baseline, the optimal temperature depends on several factors. It is a decision based on material science and process engineering.
The Material is Key
Different metals and alloys have different crystal structures and therefore different rates of hydrogen diffusion. The 200 °C value is well-established for ferritic steels. Other materials, such as certain high-strength stainless steels or nickel-based alloys, may require different temperature and time parameters to achieve effective hydrogen removal.
The 200 °C Minimum Explained
For many common steels used in construction, automotive, and industrial applications, 200 °C (approximately 400 °F) is the practical minimum temperature for a hydrogen bake-out. At this point, the diffusion rate becomes significant enough to remove damaging hydrogen within a reasonable timeframe (typically several hours).
Beyond Hydrogen Removal
It's important to distinguish this process from other heat treatments that also use hydrogen. High-temperature annealing (often above 1000 °C) in a pure hydrogen atmosphere is used for bright annealing, a process designed to reduce surface oxides and produce a clean, bright finish, not primarily to remove internal hydrogen.
Understanding the Trade-offs and Risks
Choosing a temperature is not just about effectiveness; it's about managing risk. An incorrect temperature can do more harm than good.
Risk of Over-Tempering
For steels that have been previously hardened and tempered, heating them again carries a risk. If the bake-out temperature exceeds the original tempering temperature, the material will soften, losing its carefully engineered hardness and strength. This is a primary constraint when treating high-strength fasteners and components.
Time vs. Temperature
There is an inverse relationship between time and temperature in diffusion. A slightly higher temperature can dramatically reduce the required bake-out time. However, this increases the risk of over-tempering. Conversely, a lower temperature is safer for the material's properties but requires a much longer process time to be effective, impacting production throughput.
Part Thickness and Geometry
Hydrogen must diffuse from the core of the component to its surface. For very thick parts, a longer time or a slightly higher temperature is necessary to ensure the hydrogen from the center has a chance to escape. The annealing parameters must be set based on the thickest cross-section of the part.
Making the Right Choice for Your Goal
The correct approach depends entirely on your primary objective for the material being treated.
- If your primary focus is preventing embrittlement in standard carbon or alloy steels: Begin with a baseline of 200-300 °C, ensuring the duration is sufficient for the component's thickest section.
- If your primary focus is maintaining maximum hardness in a heat-treated component: Use the lowest effective temperature possible (often just above 200 °C) and compensate with a longer process time to avoid over-tempering.
- If your primary focus is ensuring reliability in a safety-critical application: Adhere strictly to industry or engineering specifications (e.g., aerospace or automotive standards), which often dictate precise time-at-temperature requirements.
Ultimately, selecting the right hydrogen annealing temperature is a calculated balance between promoting diffusion and preserving the essential properties of your material.
Summary Table:
| Key Factor | Influence on Annealing Temperature |
|---|---|
| Material Type | Different alloys (e.g., steel vs. nickel) have different diffusion rates. |
| Goal (e.g., Prevent Embrittlement) | Baseline is often 200-300°C; higher temperatures speed up the process but risk property changes. |
| Risk of Over-Tempering | For hardened parts, temperature must not exceed the original tempering temperature. |
| Part Thickness | Thicker sections may require longer times or slightly higher temperatures for effective hydrogen removal from the core. |
Optimize your hydrogen annealing process with KINTEK.
Choosing the correct temperature is critical for preventing hydrogen embrittlement without compromising your material's hardness or strength. Our experts understand the precise balance required for different alloys and component geometries.
We provide the lab equipment and consumables you need to ensure a reliable, effective process. Let us help you achieve consistent, high-quality results for your safety-critical applications.
Contact our specialists today to discuss your specific hydrogen annealing requirements and find the right solution for your laboratory.
Visual Guide
Related Products
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vertical Laboratory Tube Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
People Also Ask
- What is hydrogen annealing? Achieve Superior Material Properties with Bright Annealing
- When would you need to use a controlled atmosphere? Prevent Contamination and Control Reactions
- What is hydrogen atmosphere heat treatment? Achieve Superior Surface Purity & Brightness
- What are the effects of hydrogen (H2) in a controlled furnace environment? Mastering Reduction and Risk
- What are hydrogen furnaces used for? Achieve Purity and Speed in High-Temperature Processing



















