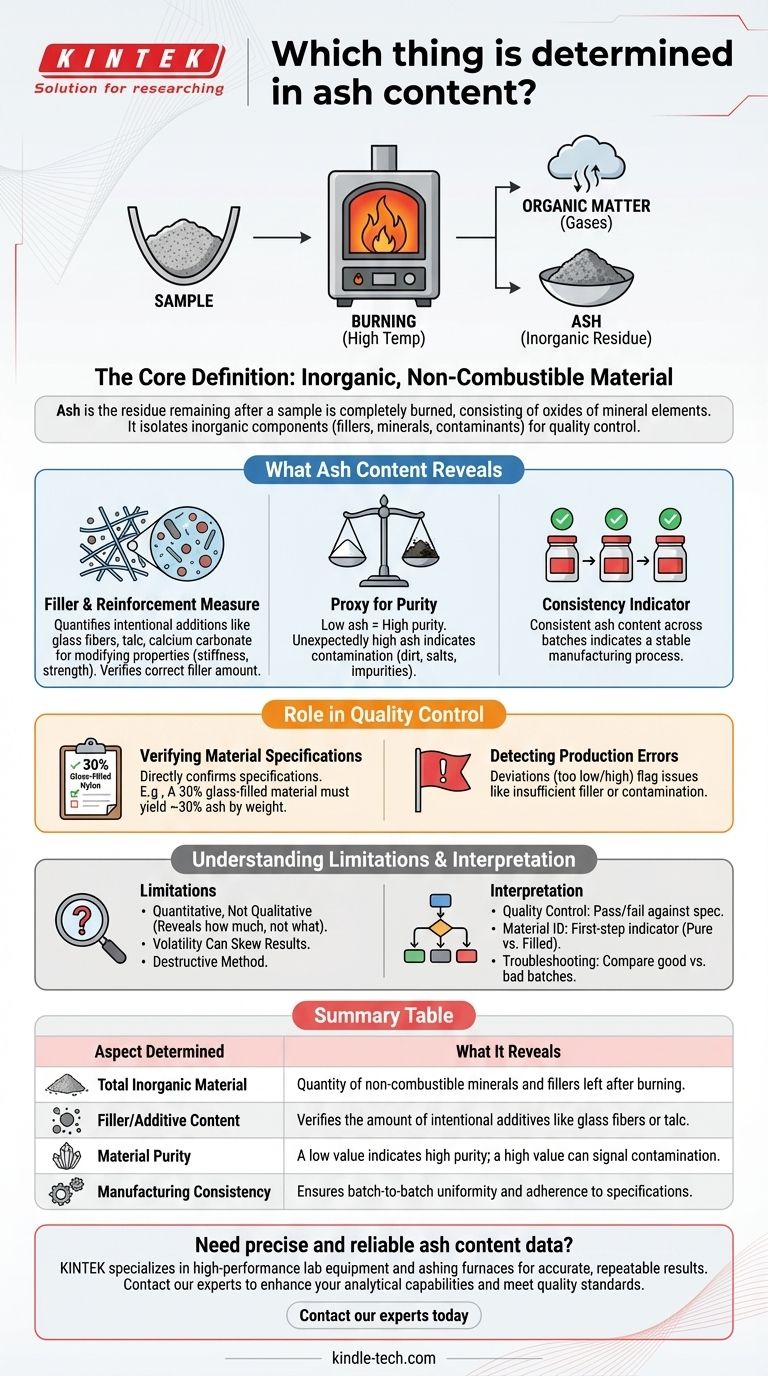
In an ash content analysis, you are determining the total amount of inorganic, non-combustible material present within a sample. After the sample is completely burned at a high temperature, the ash is the residue that remains, typically consisting of the oxides of mineral elements that were part of the original material.
The measurement of ash content is fundamentally a tool for quality control and material characterization. It isolates the inorganic components—such as fillers, minerals, and contaminants—providing a critical indicator of a product's composition, purity, and adherence to specifications.

What Ash Content Reveals About a Material
Understanding ash content is less about the ash itself and more about what its presence implies about the original material before it was burned. It serves as a powerful proxy for several key attributes.
The Core Definition: Inorganic Residue
Ash is the physical remainder of any inorganic substances in a product after all the organic matter (compounds of carbon) has been burned away.
This process effectively separates the organic fraction (which combusts into gases) from the stable, non-combustible mineral fraction.
A Direct Measure of Fillers and Reinforcements
In many industries, especially plastics and composites, inorganic materials are intentionally added to modify properties. Ash content measurement directly quantifies these additions.
Common examples include glass fibers, talc, calcium carbonate, and clay, which are added to polymers to enhance stiffness, strength, or reduce cost. The ash test verifies that the correct amount of this filler is present.
A Proxy for Purity and Consistency
For materials that are supposed to be pure, a low ash content is desirable. An unexpectedly high value can indicate contamination with dirt, salts, or other inorganic impurities.
Conversely, for filled materials, consistent ash content from batch to batch is a primary indicator of a stable manufacturing process.
The Role of Ash Content in Quality Control
The true value of an ash test lies in its application as a simple, reliable metric for ensuring quality and verifying composition.
Verifying Material Specifications
Many technical data sheets for materials will specify an ash or filler content. For example, a "30% glass-filled nylon" must be validated.
An ash test is the most direct way to confirm this specification. A sample is weighed, burned, and the remaining ash is weighed. If the ash constitutes approximately 30% of the original weight, the material meets the spec.
Detecting Production Errors
A deviation from the expected ash content is a clear red flag.
An ash value that is too low suggests that not enough filler was added, potentially leading to a weaker final product. A value that is too high could mean too much filler was added or that contamination occurred.
Understanding the Limitations
While incredibly useful, the ash content test provides specific information and it's critical to understand what it does not tell you.
It's Quantitative, Not Qualitative
An ash test reveals how much inorganic material is present but does not identify what that material is.
For example, a 15% ash content could be from talc, calcium carbonate, or a mix of contaminants. Further analytical methods, like spectroscopy, would be needed for chemical identification.
Volatility Can Skew Results
While most inorganic materials are stable, some can degrade or volatilize at the high temperatures used in ashing furnaces. This can lead to the ash weight being slightly lower than the true total inorganic content.
It Destroys the Sample
Ashing is a destructive testing method. The original sample is completely incinerated and cannot be used for any other form of analysis afterward.
How to Interpret Ash Content Results
Your interpretation of the result depends entirely on your objective and the material you are testing.
- If your primary focus is quality control: Use ash content as a pass/fail metric against a known specification for filler or mineral content.
- If your primary focus is material identification: Use ash content as a first-step indicator. A result near 0% suggests a pure, unfilled polymer, while a result of ~30% could point to a standard glass-filled grade.
- If your primary focus is troubleshooting: Compare the ash content of a "good" batch to a "bad" batch to quickly determine if inorganic composition is a contributing factor to the problem.
Ultimately, understanding ash content allows you to make data-driven decisions about your material's composition and quality.
Summary Table:
| Aspect Determined | What It Reveals |
|---|---|
| Total Inorganic Material | Quantity of non-combustible minerals and fillers left after burning. |
| Filler/Additive Content | Verifies the amount of intentional additives like glass fibers or talc. |
| Material Purity | A low value indicates high purity; a high value can signal contamination. |
| Manufacturing Consistency | Ensures batch-to-batch uniformity and adherence to specifications. |
Need precise and reliable ash content data for your quality control?
At KINTEK, we specialize in providing high-performance lab equipment, including robust ashing furnaces and consumables, designed to deliver accurate and repeatable results for your laboratory. Whether you're verifying filler content in polymers, checking food purity, or ensuring material consistency, our solutions help you maintain the highest quality standards.
Let us help you enhance your analytical capabilities. Contact our experts today to find the perfect equipment for your specific application and ensure your materials meet every specification.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What is maintenance and how can you maintain the laboratory? Boost Lab Reliability & Data Integrity
- What are the uses of furnace in chemistry laboratory? Unlock High-Temperature Material Synthesis and Analysis
- How is the sintering temperature related to the melting temperature? A Guide to Solid-State Bonding
- What is the operating temperature of the muffle furnace? Find Your Ideal Range for Lab Success
- What is a muffle furnace and how does it work? Achieve Clean, High-Temperature Heating for Your Lab



















