It’s a scenario familiar to too many researchers and engineers: your new high-temperature furnace, a significant investment meant to accelerate your work, suddenly goes cold. The heating element is visibly damaged, your critical experiment is ruined, and your project timeline is now in serious jeopardy. You’re left staring at a very expensive, very inert box, wondering, "What went wrong?"
The Expensive Cycle of Blame and Replacement
If this has happened to you, your first instinct was likely to blame the equipment. "Was it a faulty part? A low-quality brand?" You might order a replacement heating element, install it, and cautiously start over, hoping it was just a fluke.
But then, it happens again.
This frustrating cycle is more than just an annoyance; it has serious business consequences:
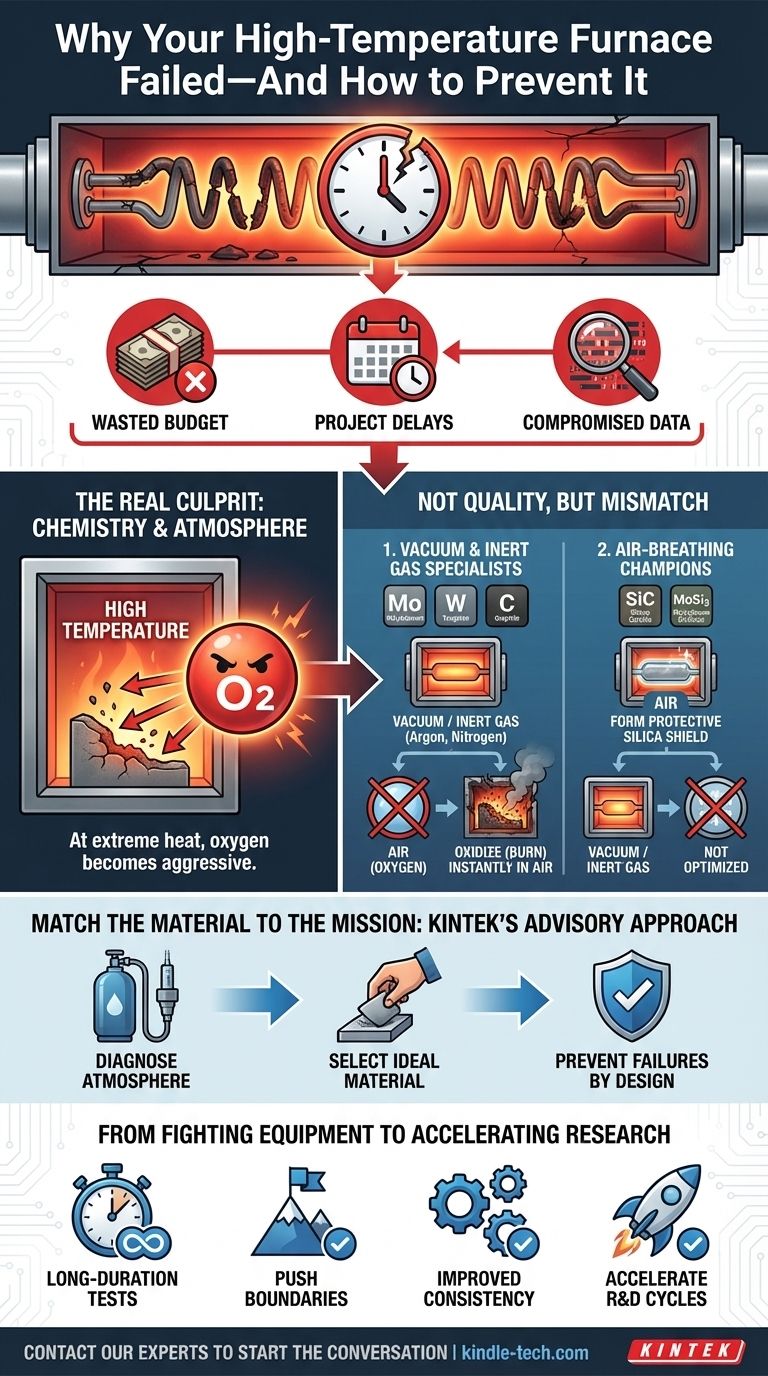
- Wasted Budget: The cost of replacement heating elements, especially for high-performance models, adds up quickly. A full furnace replacement can derail a department's budget for the year.
- Project Delays: Each failure means weeks of downtime, pushing back R&D milestones, delaying product launches, and putting you behind schedule.
- Compromised Data: Inconsistent furnace performance can cast doubt on the reliability of your experimental results, forcing you to repeat work you thought was complete.
Many labs get stuck in this loop, treating the symptom—a burnt-out element—without ever diagnosing the underlying disease. The good news is, the cause is often surprisingly simple, and understanding it is the key to breaking the cycle for good.
The Real Culprit Isn't Quality—It's Chemistry
The premature death of a furnace heating element is rarely due to a manufacturing defect. More often, it’s the result of a fundamental mismatch between the element’s material and its operating environment. The problem isn't the furnace; it's the air inside it.
At extreme temperatures, chemistry accelerates dramatically. The oxygen in the air, normally harmless, becomes a highly aggressive agent. This is where a critical distinction in furnace materials comes into play.
A Tale of Two Material Families
High-temperature heating elements generally fall into two categories, defined by how they interact with oxygen:
-
The Vacuum & Inert Gas Specialists (Molybdenum, Tungsten, Graphite): These materials are incredible performers, capable of reaching extremely high temperatures with excellent stability. However, they have an Achilles' heel: oxygen. When heated in the presence of air, they oxidize—or burn—almost instantly. Think of it like rust on hyper-speed. They are specifically designed and must be used in a vacuum or an inert gas atmosphere (like argon or nitrogen) to protect them.
-
The Air-Breathing Champions (Silicon Carbide - SiC, Molybdenum Disilicide - MoSi2): These advanced ceramics are engineered for use in air. When heated, they cleverly react with oxygen to form a thin, stable, protective layer of glass-like silica on their surface. This layer acts as a shield, preventing further oxidation and allowing the element to operate for thousands of hours at high temperatures in a normal atmosphere.
The common failure scenario—a burnt-out molybdenum element—is often just a case of running a vacuum-specialist furnace in air. The element performs exactly as designed, but in the wrong arena. Replacing it with the same material will only yield the same result.
Matching the Material to the Mission: The Key to Furnace Longevity
To truly solve this problem, you don't need a "better" furnace; you need the right furnace. You need a tool chosen not just for its maximum temperature, but for its fundamental compatibility with your process atmosphere.
This is where a deep understanding of material science becomes a practical necessity. As specialists in laboratory equipment, we at KINTEK build our advisory approach on this exact principle. Our role isn't just to sell you a furnace; it's to ensure the furnace you get is precisely configured for the work you do.
Our product range is diverse for a reason. It reflects the reality that there is no one-size-fits-all solution:
- For high-vacuum or inert-gas applications, like sintering sensitive metal powders or crystal growth, we guide you to a furnace with Molybdenum or Tungsten elements and graphite insulation—the specialists built for an oxygen-free world.
- For processes in air, like ceramic binder burnout or oxidizing heat treatments, we recommend a furnace equipped with robust Silicon Carbide (SiC) or MoSi2 elements that thrive in an oxygen environment.
By diagnosing your needs first—starting with the crucial question of atmosphere—we help you select the ideal tool from the outset. This isn't a happy accident; it's a solution designed from a deep understanding of the problem, preventing the costly failures before they ever happen.
From Fighting Your Equipment to Accelerating Your Research
Once you have a furnace that is fundamentally matched to your process, the dynamic in your lab changes. You're no longer wasting time and resources fighting your equipment. That energy is freed up, unlocking new potential.
Now, you can:
- Run long-duration tests with the confidence that your equipment is stable and reliable.
- Explore more demanding process parameters to push the boundaries of your materials research.
- Improve the consistency and yield of your production processes, from sintering to annealing.
- Accelerate your R&D cycles and bring new innovations to market faster.
Ultimately, the right furnace becomes a reliable partner in your work, not an obstacle. It enables you to focus on your true goal: discovery and innovation.
Your laboratory challenges are unique, and your equipment should be a perfect fit. Instead of guessing which furnace might survive your process, let our expertise guide you to the one designed to master it. Whether you're sintering advanced ceramics, developing new alloys, or ensuring production quality, the right material choice is the foundation of your success. Let's discuss the specifics of your project. Contact Our Experts to start the conversation.
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Molybdenum Vacuum Heat Treat Furnace
Related Articles
- The Architecture of Isolation: Anatomy of a Tube Furnace
- High Pressure Tube Furnace: Applications, Safety, and Maintenance
- The Anatomy of Control: Why Every Component in a Tube Furnace Matters
- From Crack to Complete: A Scientist's Guide to Eliminating Catastrophic Tube Furnace Failures
- Beyond Heat: The Tube Furnace as a Controlled Micro-Environment




















