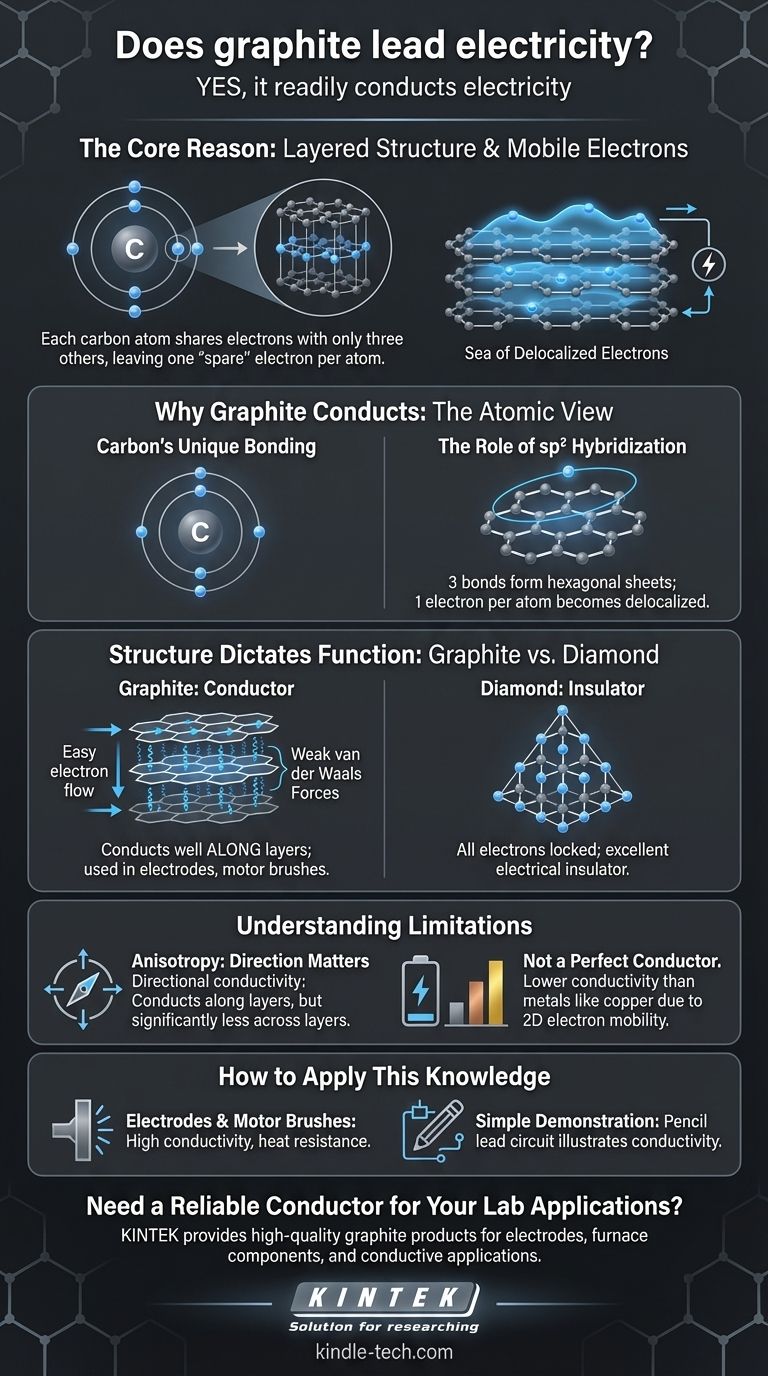
Yes, graphite readily conducts electricity. It is one of the few non-metals that is an effective electrical conductor, a property that stems directly from its unique atomic structure and the behavior of its electrons.
The core reason graphite conducts electricity is its layered structure. Each carbon atom shares electrons with only three other atoms, leaving one electron per atom free to move along these layers, creating a "sea" of mobile electrons that can carry an electrical current.

Why Graphite Conducts Electricity: The Atomic View
To understand graphite's conductivity, we must look at how its carbon atoms are arranged and bonded. The explanation lies in a concept called electron delocalization.
Carbon's Unique Bonding
A carbon atom has four outer-shell electrons (valence electrons) available for bonding. In many carbon compounds, like methane or diamond, all four of these electrons form strong, localized bonds.
The Role of sp2 Hybridization
In graphite, however, each carbon atom uses only three of its four valence electrons to form strong covalent bonds with three neighboring atoms. This arrangement, known as sp2 hybridization, creates a flat, hexagonal lattice, much like chicken wire.
A "Sea" of Delocalized Electrons
This leaves one electron per carbon atom unbound. These "spare" electrons are not locked between two specific atoms; instead, they become delocalized, forming a free-moving cloud of electrons across the entire plane of the hexagonal sheet. When a voltage is applied, these mobile electrons flow, creating an electrical current.
Structure Dictates Function: Graphite vs. Diamond
The contrast between graphite and diamond, both pure forms of carbon, provides the clearest illustration of how atomic structure determines electrical properties.
The Layered Lattice of Graphite
Graphite is essentially stacked sheets of these hexagonal carbon layers (now known as graphene). The delocalized electrons move with ease along these layers, making graphite highly conductive in that direction.
The layers themselves are held together by much weaker forces (van der Waals forces), which is why graphite feels slippery and is used as a lubricant.
The Rigid Fortress of Diamond
In a diamond, every carbon atom uses all four of its valence electrons to bond with four other carbon atoms in a rigid, three-dimensional tetrahedral lattice. This is known as sp3 hybridization.
Because all electrons are locked into strong, localized covalent bonds, there are no mobile electrons to carry a current. This makes diamond an excellent electrical insulator.
Understanding the Limitations
While graphite is a conductor, its properties are not uniform, and it doesn't behave exactly like a typical metal.
Anisotropy: Direction Matters
Graphite is an anisotropic conductor. This means its conductivity is directional. It conducts electricity extremely well along its layers but is significantly less conductive across the layers. The weak bonds between layers act as a barrier to electron flow.
Not a Perfect Conductor
While it is a good conductor for a non-metal, its conductivity is generally lower than that of metals like copper, silver, or gold. This is because metals have a three-dimensional sea of electrons, whereas graphite's electron mobility is primarily two-dimensional.
How to Apply This Knowledge
Understanding graphite's properties allows for its use in a wide range of applications, from everyday pencils to high-tech electronics.
- If your primary focus is creating electrodes or motor brushes: Graphite's combination of high conductivity, heat resistance, and self-lubricating properties makes it an ideal choice.
- If your primary focus is in advanced materials: Recognize that a single layer of graphite is graphene, a material with extraordinary electrical and mechanical properties at the nanoscale.
- If your primary focus is a simple demonstration of conductivity: A common pencil "lead" (which is a mix of graphite and clay) can be used to draw a conductive circuit on paper, illustrating the principle in a safe, accessible way.
Ultimately, graphite is a perfect example of how a material's atomic arrangement dictates its real-world function.
Summary Table:
| Property | Graphite | Diamond |
|---|---|---|
| Electrical Conductivity | Good Conductor (along layers) | Excellent Insulator |
| Atomic Bonding | sp2 Hybridization (3 bonds) | sp3 Hybridization (4 bonds) |
| Electron Behavior | Delocalized electrons move along layers | All electrons are locked in bonds |
| Structure | Layered, hexagonal sheets | Rigid, 3D tetrahedral lattice |
Need a Reliable Conductor for Your Lab Applications?
Graphite's unique properties make it an essential material for electrodes, furnace components, and other high-temperature, conductive applications.
At KINTEK, we specialize in providing high-quality lab equipment and consumables, including graphite products tailored to meet the rigorous demands of your laboratory. Our expertise ensures you get the right materials for precise temperature control, uniform heating, and long-lasting performance.
Enhance your lab's efficiency and reliability—contact our experts today to find the perfect solution for your needs!
Visual Guide
Related Products
- Carbon Graphite Plate Manufactured by Isostatic Pressing Method
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Carbon Graphite Boat -Laboratory Tube Furnace with Cover
- Large Vertical Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- How can different materials have different heat capacity? Unlocking the Microscopic Secrets of Energy Storage
- What is the purpose of laminating? Protect and Enhance Your Documents for Long-Term Use
- What role does convection play in heat transfer? Understanding Heat Movement in Fluids
- What are the applications of radioactive substances? From Medical Imaging to Nuclear Power
- What are the advantages disadvantages and uses of sheet metal? The Ultimate Guide to Material Selection



















