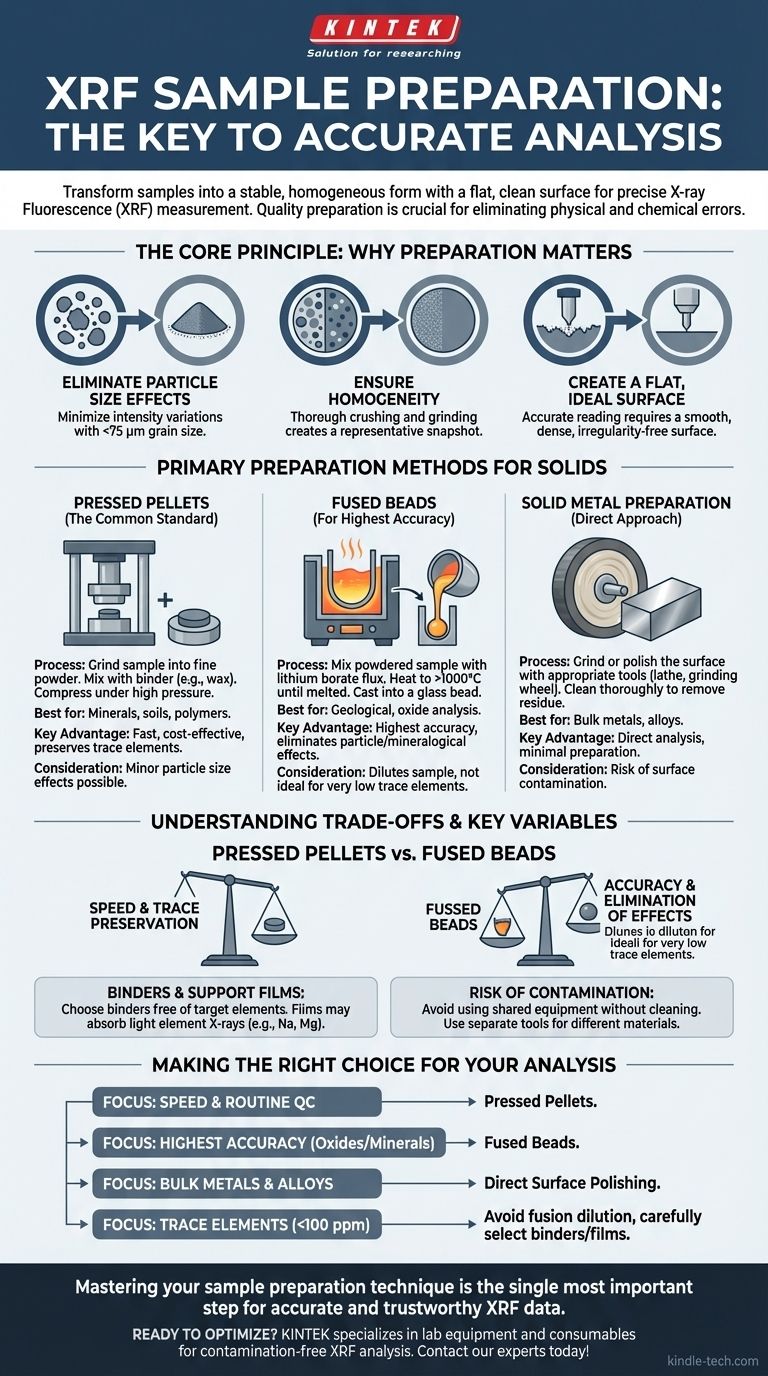
In short, samples are prepared for X-ray fluorescence (XRF) analysis by transforming them into a stable, homogeneous form with a flat, clean surface for measurement. The most common methods involve crushing and grinding the material into a fine powder, which is then either pressed into a solid pellet or fused with a flux into a glass-like bead.
The quality of your XRF results is determined more by your sample preparation than by any other factor. The goal is not just to prepare a sample, but to create one that is a perfectly uniform and representative snapshot of the original bulk material, thereby eliminating physical and chemical sources of error.

The Core Principle: Why Preparation is Critical
Before detailing the methods, it is essential to understand why preparation is so crucial for accurate XRF analysis. The instrument measures a very small volume of the sample's surface, so that surface must perfectly represent the entire material.
Eliminating Particle Size Effects
The intensity of the fluorescent X-rays can be affected by the size, shape, and packing of the grains in a sample.
Grinding the sample to a very fine and uniform powder—typically smaller than 75 micrometers (µm)—minimizes these particle size effects and ensures the measurement is consistent.
Ensuring a Representative and Homogeneous Sample
Most materials are not naturally homogeneous. Crushing and grinding mix the material thoroughly, ensuring that the small portion analyzed by the XRF is chemically identical to the bulk material.
Creating a Flat, Ideal Surface
The XRF instrument's geometry requires a perfectly flat surface for an accurate reading. Any roughness or irregularity can scatter X-rays and skew the results, making a smooth, dense surface a non-negotiable requirement.
Primary Preparation Methods for Solids
While liquids and loose powders can be analyzed, the highest quality data for solid materials comes from creating pellets, beads, or polished surfaces.
Method 1: Pressed Pellets (The Common Standard)
This is the most popular method due to its balance of speed, cost, and quality. It is excellent for a wide range of materials, from minerals and soils to polymers.
The process involves grinding the sample into a fine powder. If the powder does not bind well on its own, a binder (like a wax powder) is mixed in. This mixture is then placed into a die and compressed under high pressure to form a durable, solid pellet.
Method 2: Fused Beads (For Highest Accuracy)
For applications demanding the highest precision and accuracy, particularly in geological or oxide analysis, creating a fused bead is the superior method.
Here, the powdered sample is mixed with a lithium borate flux. The mixture is then heated in a crucible to over 1000°C until it melts, completely dissolving the sample. The molten glass is then cast into a perfectly flat, solid bead. This process completely eliminates particle size and mineralogical effects.
Method 3: Solid Metal Preparation
For analyzing solid metals and alloys, the approach is much more direct. The goal is simply to create a clean and flat surface on the bulk material itself.
This is typically achieved by grinding or polishing the surface with appropriate tools, like a lathe for soft metals or a grinding wheel for hard alloys. The surface must then be cleaned to remove any residue or contamination from the preparation process.
Understanding the Trade-offs and Key Variables
Choosing the right method requires understanding the compromises involved and the factors that can introduce errors into your analysis.
Pressed Pellets vs. Fused Beads
Pressed pellets are fast and preserve the concentration of trace elements. However, they are still susceptible to minor particle size and mineralogical effects.
Fused beads eliminate these physical effects entirely, yielding higher accuracy. The main trade-off is that the flux dilutes the sample, which can make it difficult to measure elements present at very low concentrations. The process is also more complex and time-consuming.
The Role of Binders and Support Films
Binders are essential for creating durable pellets from non-cohesive powders, but they also dilute the sample. You must choose a binder that does not contain any of the elements you are trying to measure.
Similarly, if a thin plastic film is used to support a loose powder, that film can absorb some of the X-rays, particularly from lighter elements, leading to inaccurate results for elements like Sodium (Na) or Magnesium (Mg).
The Risk of Contamination
Contamination is a constant risk during preparation. Using grinding equipment previously used for a different sample type can introduce foreign elements. Likewise, using separate files for cleaning different metal alloys is critical to prevent cross-contamination.
Making the Right Choice for Your Analysis
Your preparation method should be selected based on your sample type, analytical goals, and accuracy requirements.
- If your primary focus is speed and routine quality control: Pressed pellets offer the best balance of speed, cost, and reliable results.
- If your primary focus is the highest possible accuracy for oxides or minerals: Fused beads are the definitive choice, as they eliminate physical matrix effects.
- If your primary focus is analyzing bulk metals or alloys: Direct surface polishing is the most efficient and appropriate method.
- If your primary focus is measuring trace elements (<100 ppm): Avoid fusion to prevent dilution, and carefully select binders and films that are free of your elements of interest.
Ultimately, mastering your sample preparation technique is the single most important step you can take to produce accurate and trustworthy XRF data.
Summary Table:
| Preparation Method | Best For | Key Advantage | Key Consideration |
|---|---|---|---|
| Pressed Pellets | Minerals, soils, polymers | Fast, cost-effective, preserves trace elements | Minor particle size effects possible |
| Fused Beads | Oxides, geological samples | Highest accuracy, eliminates matrix effects | Dilutes sample, not ideal for trace elements |
| Solid Polishing | Metals, alloys | Direct analysis, minimal preparation | Risk of surface contamination |
Ready to optimize your XRF sample preparation? KINTEK specializes in lab equipment and consumables for precise, contamination-free XRF analysis. Whether you need pellet presses, fusion flux, or polishing tools, our solutions ensure your samples are perfectly prepared for accurate results. Contact our experts today to discuss your laboratory needs!
Visual Guide
Related Products
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Double Plate Heating Press Mold for Lab
People Also Ask
- What kind of steel is used in hydraulic press? Engineering High-Strength Steel for Extreme Force
- What is the purpose of applying high pressure in dry cathode preparation? Achieve Peak Solid-State Battery Density
- What is the pressed pellet technique for XRF? A Guide to Accurate Sample Preparation
- Why is a laboratory hydraulic press used for 380 MPa pressure? Master Solid-State Electrolyte Densification
- Why is it necessary to process nickel ore powder into pellets? Optimize Gas Permeability for Reductive Roasting
- What is the difference between hydraulic and mechanical press used in forging? Choose the Right Press for Your Production Needs
- What are the primary applications of a Laboratory Hydraulic Press in Na3OBr precursor preparation? Enhance Synthesis.
- Is a hydraulic press safe? How to Mitigate Crushing, Injection, and Failure Risks



















