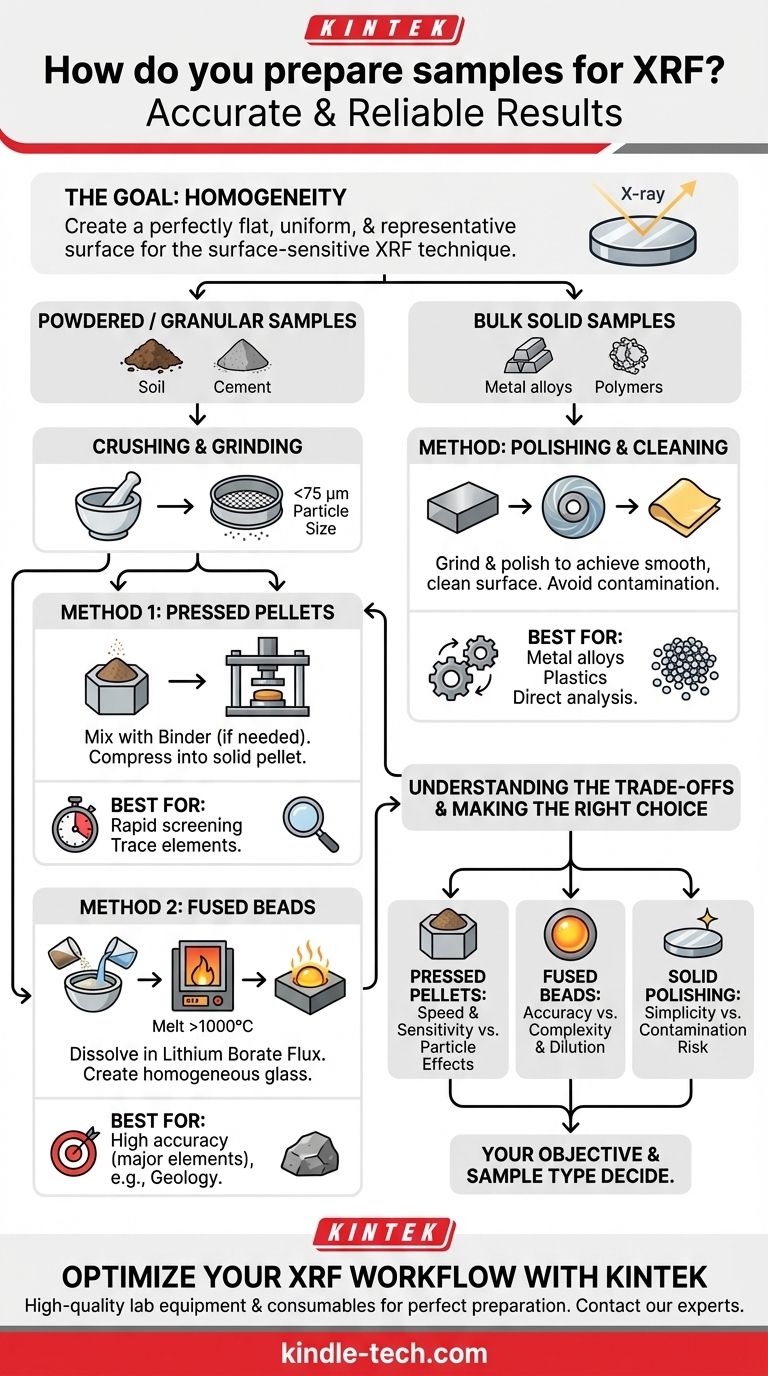
The most common methods for preparing samples for X-ray Fluorescence (XRF) analysis involve transforming the material into a robust, homogeneous form with a flat, clean surface. This is typically achieved by grinding materials into a fine powder and pressing them into a pellet, fusing the powder with a flux to create a glass-like bead, or simply polishing a bulk solid material to the required smoothness.
The fundamental goal of any XRF sample preparation method is to present a perfectly flat, uniform, and representative surface to the instrument's X-ray beam. How you achieve this depends entirely on the nature of your sample and the level of analytical accuracy you require.

Why Sample Preparation is Critical for XRF
XRF is a Surface-Sensitive Technique
X-ray Fluorescence analysis primarily interrogates the top layer of a sample. The instrument's primary X-ray beam penetrates only a shallow depth into the material.
Therefore, the quality of your results is directly dictated by the quality of this surface. Any imperfections, contamination, or non-uniformity will produce inaccurate and unreliable data.
The Goal: Homogeneity
The ideal sample is homogeneous, meaning it has the same composition throughout. Proper preparation eliminates issues like inconsistent particle size or uneven distribution of elements, ensuring the analyzed surface is truly representative of the entire sample.
Preparing Powdered or Granular Samples
For materials that are brittle, granular, or can be ground (like minerals, soils, and cement), the goal is to create a uniform powder that can be formed into a dense, flat disc.
The First Step: Crushing and Grinding
Nearly all powder-based methods begin with reducing the sample to a fine, consistent grain size. The standard target is a particle size of less than 75 micrometers (µm).
This step is crucial for minimizing what are known as "particle size effects," where larger grains can disproportionately affect the X-ray signal.
Method 1: Pressed Pellets
This is a fast, low-cost, and widely used method for a variety of sample types. The finely ground powder is poured into a die and compressed under high pressure to form a solid, stable pellet.
If a powder does not bind well on its own, a wax binder is often mixed in to help the particles cohere into a robust pellet.
Method 2: Fused Beads
For the highest level of accuracy, particularly for major elements, fusion is the preferred method. The sample is mixed with a lithium borate flux and heated in a crucible to over 1000°C.
This process dissolves the sample completely, creating a perfectly homogeneous molten glass that is then cast into a flat, stable bead. This eliminates all particle size and mineralogical effects.
Preparing Bulk Solid Samples
For materials that are already in a solid form, such as metal alloys, plastics, or polymers, the preparation process is much simpler.
The Core Requirement: A Flat, Clean Surface
The primary objective is to create a measurement surface that is smooth and free of any contamination. The preparation method depends on the material's hardness.
The Process: Polishing and Cleaning
Hard metals are typically prepared using grinding tools to achieve a smooth finish. Softer metals may be finished on a lathe.
Crucially, the surface must be cleaned and handled carefully to avoid contamination. It is essential to use separate files or polishing media for different sample types to prevent cross-contamination.
Understanding the Trade-offs
No single method is perfect for every situation. The choice involves balancing speed, cost, and the required analytical precision.
Pressed Pellets: Speed vs. Particle Effects
This method is excellent for its speed and low cost, making it ideal for high-throughput screening. However, it can still be susceptible to minor inaccuracies from residual particle size or mineralogical effects if not prepared carefully.
Fused Beads: Accuracy vs. Complexity & Dilution
Fusion provides the most accurate and repeatable results for major elemental concentrations by eliminating physical effects. The main drawback is that the sample is diluted by the flux, which can make analyzing trace elements (at very low concentrations) more difficult. The process is also slower and more complex.
Solid Polishing: Simplicity vs. Contamination Risk
Directly analyzing a solid is the simplest method when applicable. However, it carries a high risk of surface contamination, and improper polishing can smear softer metals, creating a surface layer that is not representative of the bulk material.
Making the Right Choice for Your Goal
Your analytical objective and sample type are the deciding factors.
- If your primary focus is rapid screening or analyzing trace elements: Pressed pellets offer the best balance of speed, cost, and sensitivity.
- If your primary focus is the highest possible accuracy for major elements (e.g., in geology or cement): Fused beads are the definitive choice, as they eliminate the physical sources of error.
- If your primary focus is analyzing a solid metal alloy or polymer: Direct analysis after proper polishing and cleaning is the most straightforward and effective method.
Choosing the correct preparation technique is the most important step in guaranteeing the quality and reliability of your XRF results.
Summary Table:
| Method | Best For | Key Advantage | Key Consideration |
|---|---|---|---|
| Pressed Pellets | Rapid screening, trace element analysis | Fast, low-cost, minimal sample dilution | Potential for minor particle size effects |
| Fused Beads | High-accuracy major element analysis (e.g., geology, cement) | Eliminates particle size/mineralogy effects | Dilutes sample, more complex and slower process |
| Solid Polishing | Metal alloys, plastics, polymers | Simple and direct | Risk of surface contamination or smearing |
Ensure Accurate and Reliable XRF Results with KINTEK
Choosing the right sample preparation method is critical for the success of your XRF analysis. The quality of your data starts with the quality of your sample preparation.
KINTEK specializes in providing the high-quality lab equipment and consumables you need for perfect XRF sample preparation every time. Whether your lab requires robust pellet presses, reliable fusion fluxers, or precise polishing tools, we have the solutions to meet your specific application—from rapid quality control screening to the most demanding research and development.
Let our experts help you optimize your workflow. We can guide you to the best preparation method for your materials and analytical goals.
Contact our team today to discuss your XRF needs and discover how KINTEK can support your laboratory's success.
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Laboratory Manual Hydraulic Pellet Press for Lab Use
People Also Ask
- How does a hydraulic press increase the force on an object? Multiply Force with Pascal's Law
- How is a hydraulic press utilized in the assembly of all-solid-state battery anodes? Optimize Indium Foil Interfaces
- What is C type and H type power press? Choose the Right Press for Precision or Accessibility
- What is a 100 ton press used for? A Guide to Industrial Bending, Forming, and Assembly
- What is the importance of XRF analysis? Unlock Accurate Elemental Composition Data
- How much force can a hydraulic press generate? Unlock Massive Power from 1 to 80,000+ Tons
- Why is a KBr pellet used? Creating a Clear Window for Accurate FTIR Analysis
- What happens when a hydraulic system overheats? Prevent Costly Damage and Downtime



















