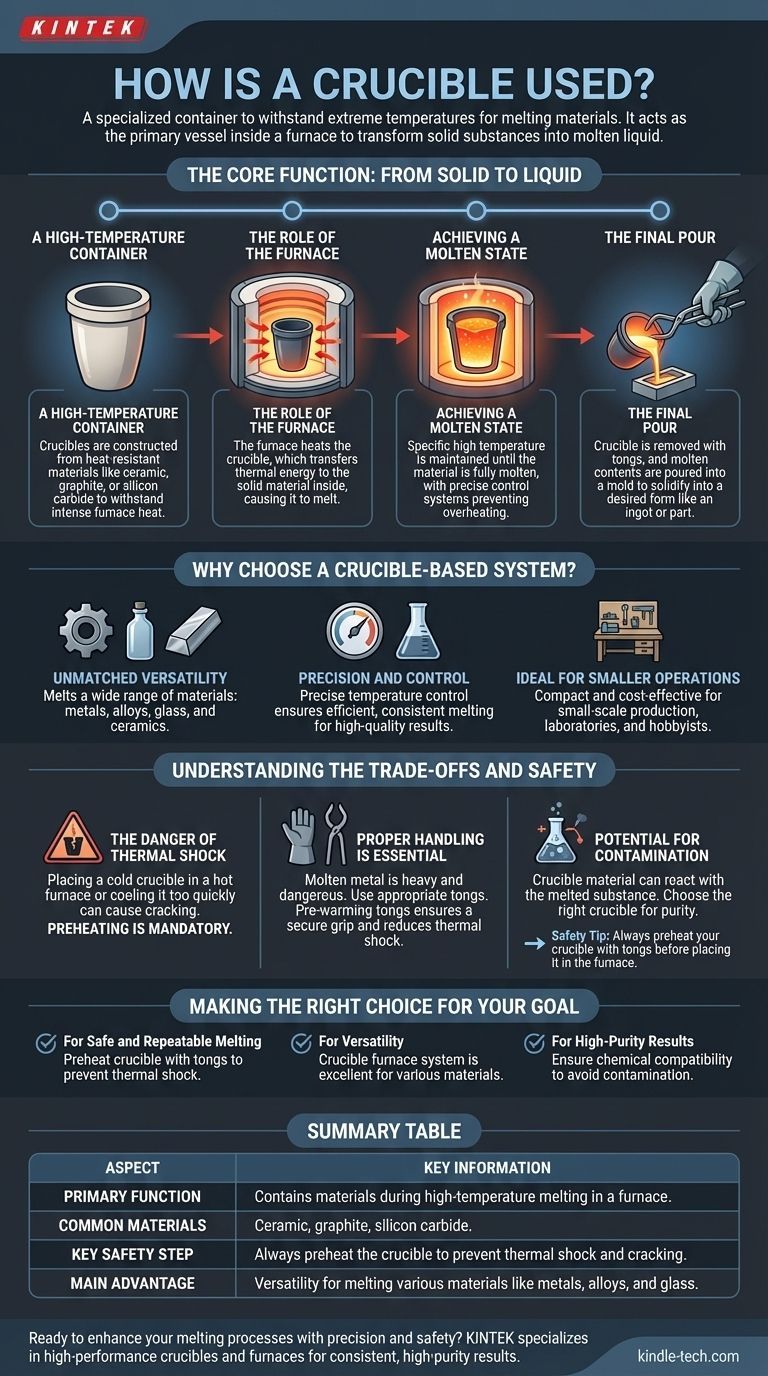
In essence, a crucible is a specialized container designed to withstand extreme temperatures for melting materials. It acts as the primary vessel placed inside a furnace, where it heats a solid substance, like metal or glass, until it becomes a molten liquid. This liquid can then be poured into a mold to create a new shape.
The core function of a crucible is to act as an intermediary, safely containing a material as it is heated to its melting point by an external source, typically a furnace, enabling its transformation from a solid to a pourable liquid for casting or analysis.

The Core Function: From Solid to Liquid
A crucible's role is simple in concept but critical in execution. It is the central component in any process that requires the controlled melting of a substance at high temperatures.
A High-Temperature Container
A crucible is not just any pot. It is constructed from materials like ceramic, graphite, or silicon carbide that have exceptionally high melting points and can resist the intense heat generated by a furnace without breaking down.
The Role of the Furnace
The crucible is placed inside a furnace (such as an electric or muffle furnace), which is the source of the heat. The furnace heats the crucible, and the crucible, in turn, transfers that thermal energy to the material inside it, causing it to melt.
Achieving a Molten State
The system maintains a specific high temperature until the material inside the crucible is fully melted. Modern crucible furnaces often include precise temperature control systems to ensure the material reaches its ideal molten state without being overheated or damaged.
The Final Pour
Once the material is liquid, the crucible is carefully removed from the furnace using specialized tongs. The molten contents are then poured into a mold or another container to cool and solidify into the desired form, such as an ingot, a piece of jewelry, or a machine part.
Why Choose a Crucible-Based System?
Using a crucible and furnace offers several distinct advantages, making it a preferred method in laboratories, foundries, and workshops.
Unmatched Versatility
Crucible furnaces are highly versatile and can be used to melt a wide range of materials. This includes various metals, alloys, glass, and ceramics, making them suitable for many different industries and applications.
Precision and Control
The ability to precisely control the temperature is a key benefit. This ensures that materials are melted efficiently and consistently, which is crucial for achieving high-quality results in casting and material science.
Ideal for Smaller Operations
Due to their often compact size and cost-effectiveness, crucible systems are an excellent choice for small-scale production, laboratory research, and hobbyist applications where large, industrial-scale melting is not required.
Understanding the Trade-offs and Safety
While effective, using a crucible requires a clear understanding of the risks and best practices involved in high-temperature work.
The Danger of Thermal Shock
One of the most critical considerations is thermal shock. Placing a cold crucible into a red-hot furnace or cooling it too quickly can cause it to crack or shatter. This is why preheating the crucible is a mandatory safety step.
Proper Handling is Essential
A crucible filled with molten metal is extremely heavy and dangerous. It must be handled with crucible tongs that are appropriately sized and in good condition. Pre-warming the tongs before gripping the crucible helps ensure a secure grip and reduces the risk of thermal shock.
Potential for Contamination
The material of the crucible itself can sometimes react with or contaminate the substance being melted. Choosing the right type of crucible for the specific material you are working with is essential for maintaining purity.
Making the Right Choice for Your Goal
Applying this knowledge depends entirely on your objective.
- If your primary focus is safe and repeatable melting: Always preheat your crucible with tongs before placing it in the furnace to prevent cracking from thermal shock.
- If your primary focus is versatility for a workshop or lab: A crucible furnace system is an excellent investment, allowing you to work with a wide array of materials from metals to glass.
- If your primary focus is achieving high-purity results: Ensure your crucible's material is chemically compatible with the substance you intend to melt to avoid contamination.
Ultimately, using a crucible is a foundational process for transforming raw materials into new and useful forms through the controlled application of heat.
Summary Table:
| Aspect | Key Information |
|---|---|
| Primary Function | Contains materials during high-temperature melting in a furnace. |
| Common Materials | Ceramic, graphite, silicon carbide. |
| Key Safety Step | Always preheat the crucible to prevent thermal shock and cracking. |
| Main Advantage | Versatility for melting various materials like metals, alloys, and glass. |
Ready to enhance your melting processes with precision and safety? KINTEK specializes in high-performance lab equipment and consumables, including crucibles and furnaces designed for reliability and exact temperature control. Whether you're in a research lab, foundry, or workshop, our solutions help you achieve consistent, high-purity results. Contact our experts today to find the perfect crucible system for your specific materials and goals!
Visual Guide
Related Products
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Custom Machined and Molded PTFE Teflon Parts Manufacturer with PTFE Crucible and Lid
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
People Also Ask
- What is a crucible material for a furnace? A Guide to Choosing the Right High-Temperature Container
- What are the benefits of using an alumina crucible with a lid for TiB2 nanopowder heat treatment? Ensure High Purity
- What is the temperature range of alumina crucibles? Key Factors for Safe High-Temp Use
- Why use high-purity alumina crucibles for RPPO calcination? Ensure Stoichiometric Purity at 1150°C
- What is the purpose of using an alumina crucible with a lid for g-C3N4 synthesis? Optimize Your Nanosheet Production



















