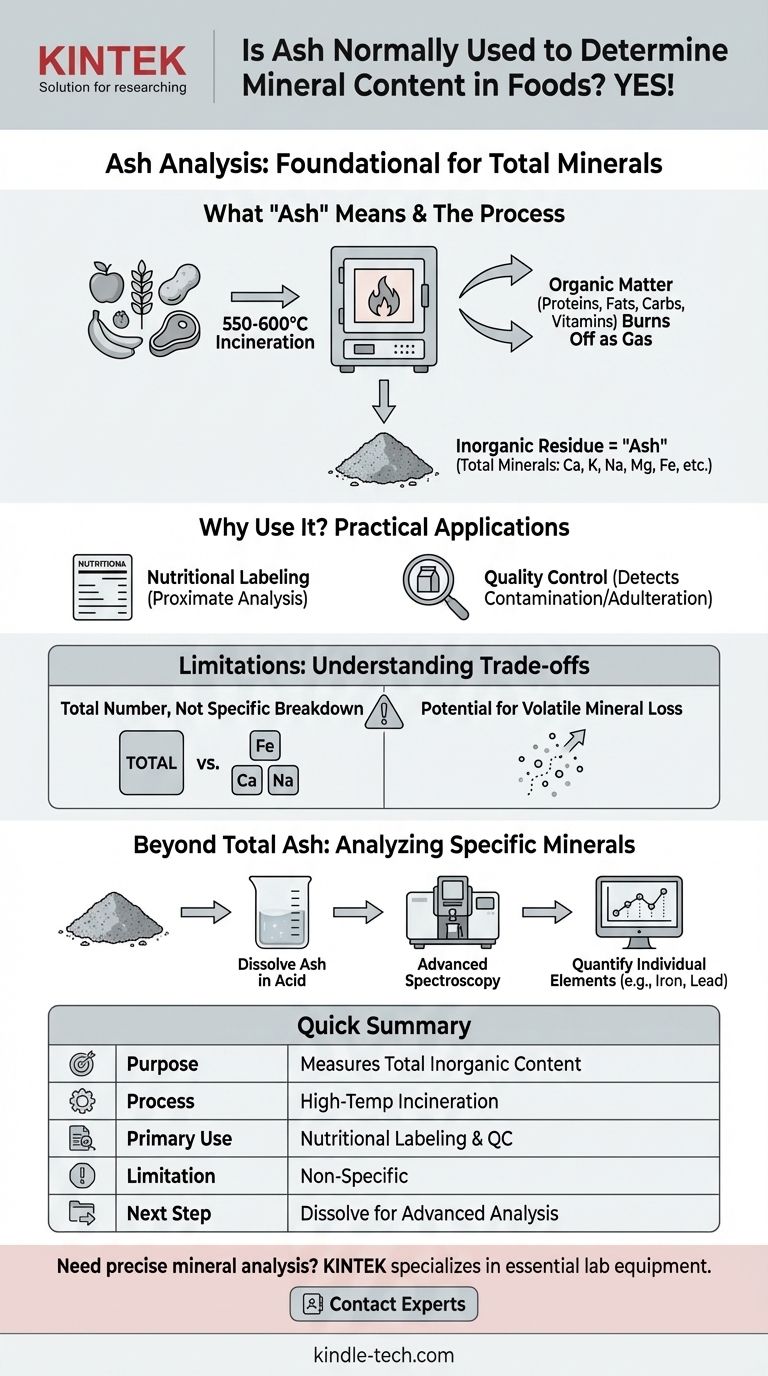
Yes, ash analysis is the foundational and most common method used to determine the total mineral content in food. This technique, known as proximate analysis, involves incinerating a food sample at a very high temperature to burn off all the organic matter—proteins, fats, carbohydrates, and vitamins. The inorganic, non-combustible residue that remains is called "ash," and its weight is a direct measure of the total mineral content.
Ash analysis provides a crucial, high-level figure: the total quantity of all minerals combined. While it does not identify individual minerals, it is the essential starting point for both nutritional assessment and quality control in the food industry.
What "Ash" Really Means in Food Science
Ash is the inorganic residue remaining after the water and organic matter have been removed by heating. It is a fundamental component in the chemical analysis of food.
The Principle of Incineration
The process involves carefully weighing a food sample and heating it in a high-temperature muffle furnace, typically between 550 and 600°C.
This intense heat causes all organic compounds to combust and turn into gases like carbon dioxide, water vapor, and nitrogen oxides, which then dissipate.
Ash as a Proxy for Total Minerals
The material left behind—the ash—is composed of the oxides, sulfates, phosphates, chlorides, and silicates of the elements that were present in the food.
These elements are the minerals we recognize, such as calcium, potassium, sodium, magnesium, and iron. Therefore, measuring the weight of the ash serves as an excellent proxy for the food's total mineral load.
The Practical Role of Ash Analysis
Determining ash content is not just an academic exercise; it has critical real-world applications in nutrition, quality, and food processing.
A Cornerstone of Nutritional Labeling
Ash is one of the six key components measured in proximate analysis, the standard method for creating a food's nutritional profile. The other five are moisture, crude protein, crude fat, crude fiber, and carbohydrates.
This total mineral value is a key part of understanding a food's overall nutritional composition.
A Critical Tool for Quality Control
The ash content of many raw materials is a well-established index of quality. For example, a higher-than-normal ash content in flour could indicate contamination with bran particles or even soil.
In fruit juices and syrups, ash measurement helps verify authenticity and detect adulteration or the use of forbidden additives.
Understanding the Trade-offs and Limitations
While foundational, ash analysis is a crude measurement. Understanding its limitations is key to interpreting the results correctly.
Lack of Specificity: A Total Number, Not a Breakdown
The primary limitation is that ash content gives you a single number representing the sum of all minerals. It cannot distinguish between beneficial minerals like iron and potentially toxic heavy metals like lead or mercury.
If you need to know the specific amount of calcium or sodium, ash analysis alone is insufficient.
The Risk of Mineral Loss During Heating
Some mineral elements and salts can be volatile at the high temperatures used for ashing.
Elements like chlorine, iodine, mercury, and selenium can be partially or completely lost during the process, leading to an underestimation of the true total mineral content.
Potential for Chemical Transformations
During incineration, minerals can react with each other or with atmospheric oxygen. For example, carbonates can form from organic salts.
These chemical changes can alter the final weight of the residue, introducing a small margin of error compared to the mineral content in the original, unheated food.
Moving Beyond Total Ash: Analyzing Specific Minerals
When the concentration of individual minerals is required, ash analysis becomes the first step in a more complex analytical workflow.
Ashing as a Preparatory Step
To measure a specific mineral like iron, the ash obtained from incineration is first dissolved in a strong acid. This creates a clear, acidic solution containing all the non-volatile minerals from the original sample.
This liquid sample is now ready for more sophisticated analysis.
Advanced Spectroscopic Techniques
Once the ash is dissolved, chemists use advanced instrumental methods to quantify individual elements.
Techniques like Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Plasma (ICP) analysis can precisely measure the concentration of dozens of different minerals within the same sample, providing the detailed breakdown that ash analysis cannot.
Making the Right Choice for Your Goal
The analytical method you choose depends entirely on the information you need.
- If your primary focus is nutritional profiling or quality control: Start with ash analysis, as it provides the essential total mineral content required for proximate analysis and serves as a key quality indicator.
- If your primary focus is quantifying specific essential minerals or contaminants: Use ash analysis as a sample preparation step, then employ advanced methods like AAS or ICP on the resulting ash to determine the concentration of individual elements.
Ultimately, understanding the purpose and limits of ash analysis is fundamental to accurate and effective food science.

Summary Table:
| Aspect | Key Information |
|---|---|
| Purpose | Measures total inorganic mineral content in food samples. |
| Process | Incineration at 550-600°C to burn off organic matter, leaving mineral residue (ash). |
| Primary Use | Foundational step for proximate analysis, nutritional labeling, and quality control. |
| Limitation | Provides total mineral weight; does not identify or quantify specific individual minerals. |
| Next Step for Specifics | Ash is dissolved and analyzed via techniques like AAS or ICP for individual mineral data. |
Need precise mineral analysis for your food products?
KINTEK specializes in supplying the high-quality lab equipment and consumables essential for accurate ash analysis and advanced mineral testing. From reliable muffle furnaces for sample preparation to the instruments needed for detailed spectroscopic analysis, we support laboratories in achieving precise nutritional and quality control data.
Enhance your lab's capabilities and ensure accurate results—contact our experts today to discuss your specific needs!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the difference between oven and muffle furnace? A Guide to Choosing the Right Heating Equipment
- What is the muffle furnace used for in metallurgy? Achieve Precise, Contaminant-Free Heat Treatment
- Can a muffle furnace be used for pyrolysis? How to adapt it for oxygen-free thermal decomposition
- What is the temperature setting of a muffle furnace? Select the Right Model for Your Process
- What is the inside of a muffle furnace? Discover the Key Components for Precise High-Temperature Processing



















