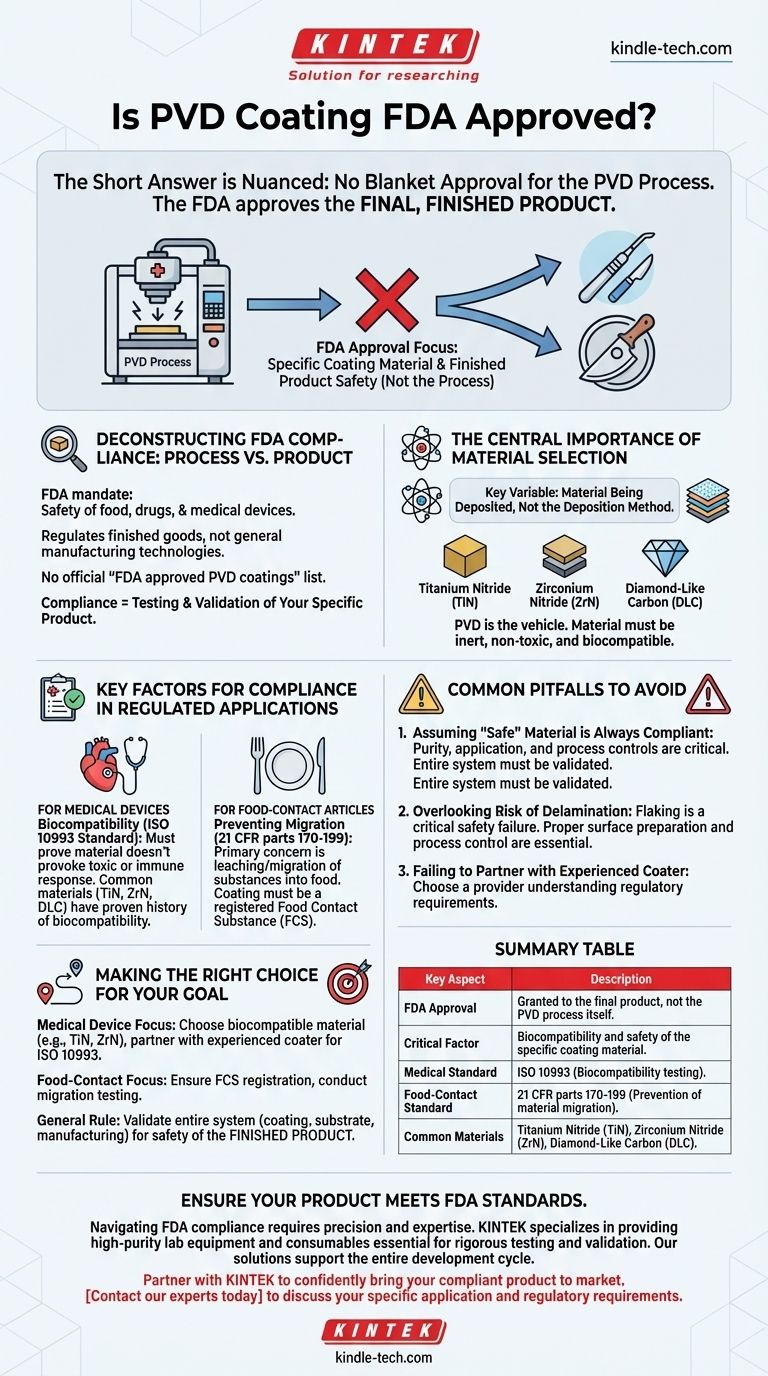
The short answer is nuanced. The U.S. Food and Drug Administration (FDA) does not grant a blanket "approval" for a manufacturing process like Physical Vapor Deposition (PVD) itself. Instead, the FDA approves the final, finished product—such as a specific medical device or food-contact item—that utilizes a PVD coating, after the manufacturer has proven its safety and efficacy for its intended use.
The critical distinction is that regulatory approval focuses on the biocompatibility and safety of the specific coating material used on a finished product, not on the PVD process in a vacuum. The responsibility for proving this safety lies entirely with the product manufacturer.

Deconstructing FDA Compliance: Process vs. Product
To navigate the regulatory landscape, you must understand that the FDA's concern is the final article that interacts with the human body or with food, not the methods used to create it.
The FDA's Role and Focus
The FDA's mandate is to ensure the safety of food, drugs, and medical devices. It regulates finished goods, not general manufacturing technologies.
Therefore, you will not find an official FDA list of "approved PVD coatings." The path to compliance is through testing and validating your specific product.
The Central Importance of Material Selection
The key variable in the compliance equation is the material being deposited, not the deposition method.
PVD is simply the vehicle for applying an extremely thin layer of material. If that material is inert, non-toxic, and biocompatible—like Titanium Nitride (TiN)—it is a strong candidate for use on a regulated product.
The Manufacturer's Burden of Proof
The company that brings the final product to market is solely responsible for conducting the necessary testing to prove its safety.
This involves submitting a comprehensive data package to the FDA that demonstrates the coated product meets all relevant standards for its intended use.
Key Factors for Compliance in Regulated Applications
Whether your product is a medical implant or a piece of food processing equipment, the core principles of safety validation are the same.
For Medical Devices: Biocompatibility is Paramount
Biocompatibility is the measure of how a material interacts with the human body. A biocompatible material does not provoke a toxic, injurious, or immunological response.
The international standard for this is ISO 10993. Any PVD-coated medical device must undergo a series of tests under this standard to prove it is safe for its specific application (e.g., surgical instrument, long-term implant).
Materials like Titanium Nitride (TiN), Zirconium Nitride (ZrN), and certain forms of Diamond-Like Carbon (DLC) are frequently used because they have a long history of being inert and biocompatible.
For Food-Contact Articles: Preventing Migration
For items that touch food, the primary concern is that the coating does not leach or migrate harmful substances into the food supply.
The coating material must be considered a Food Contact Substance (FCS) and comply with relevant FDA regulations, such as those found in 21 CFR parts 170-199.
Again, the manufacturer must conduct testing to prove that no unsafe level of material transfers from the coated surface to the food under expected conditions of use.
Common Pitfalls to Avoid
Achieving compliance requires careful planning and avoiding common assumptions that can lead to costly delays or rejections.
Assuming a "Safe" Material is Always Compliant
The purity and application of the material are critical. A coating of nominally "safe" TiN can be rendered non-compliant if the PVD process introduces contaminants or does not achieve proper adhesion.
You must validate the entire system: the substrate material, the cleaning process, the coating material, and the deposition parameters.
Overlooking the Risk of Delamination
A primary failure mode for any coating is delamination, or flaking off the surface. For a medical implant or a food-grade surface, this is a critical safety failure.
Proper surface preparation and process control are essential to ensure the coating has a powerful, permanent bond to the underlying product. This adhesion must be verified through rigorous testing.
Failing to Partner with an Experienced Coater
Work with a PVD coating provider who understands the regulatory requirements for your industry. They should be able to provide documentation on material purity, process controls, and may even have a Master File with the FDA that you can reference in your submission.
Making the Right Choice for Your Goal
To ensure regulatory success, your approach must be deliberate and focused on validating your final product.
- If your primary focus is a medical device: Select a coating material with a proven history of biocompatibility (e.g., TiN, ZrN) and partner with a coater who has experience qualifying products to ISO 10993 standards.
- If your primary focus is a food-contact article: Ensure the coating material is a registered Food Contact Substance and conduct the necessary migration testing on your final product to prove it meets FDA safety standards.
- For any regulated application: Remember that you are responsible for validating the entire system—the coating, the substrate, and the manufacturing process—to prove the safety of your specific end product.
Ultimately, FDA compliance is achieved not by choosing a pre-approved coating, but by rigorously demonstrating the safety of your finished product for its intended use.
Summary Table:
| Key Aspect | Description |
|---|---|
| FDA Approval | Granted to the final product, not the PVD process itself. |
| Critical Factor | Biocompatibility and safety of the specific coating material. |
| Medical Standard | ISO 10993 (Biocompatibility testing). |
| Food-Contact Standard | 21 CFR parts 170-199 (Prevention of material migration). |
| Common Materials | Titanium Nitride (TiN), Zirconium Nitride (ZrN), Diamond-Like Carbon (DLC). |
Ensure Your Product Meets FDA Standards
Navigating FDA compliance for a PVD-coated medical device or food-contact article requires precision and expertise. KINTEK specializes in providing high-purity lab equipment and consumables essential for the rigorous testing and validation your product needs. Our solutions support the entire development cycle, from material selection to final product safety verification.
Partner with KINTEK to confidently bring your compliant product to market. Contact our experts today to discuss your specific application and regulatory requirements.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Aluminized Ceramic Evaporation Boat for Thin Film Deposition
People Also Ask
- How does RF power create plasma? Achieve Stable, High-Density Plasma for Your Applications
- Why does PECVD commonly use RF power input? For Precise Low-Temperature Thin Film Deposition
- What are the benefits of PECVD? Achieve Superior Low-Temperature Thin Film Deposition
- How are PECVD and CVD different? A Guide to Choosing the Right Thin-Film Deposition Process
- What are the advantages of PECVD? Enable Low-Temperature, High-Quality Thin-Film Deposition



















