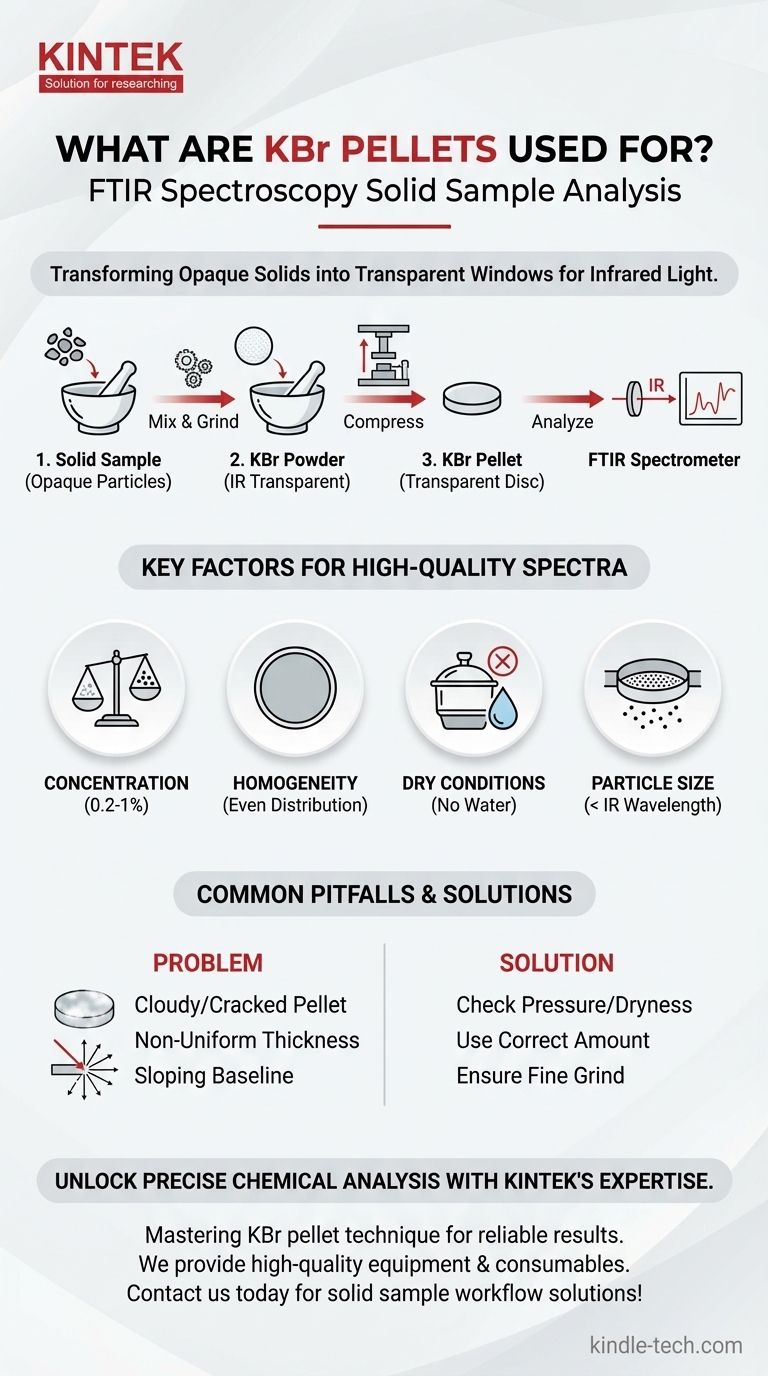
In analytical chemistry, KBr pellets are a standard and essential method for preparing solid samples for analysis via Fourier Transform Infrared (FTIR) spectroscopy. This technique involves mixing a small amount of a solid sample with pure potassium bromide (KBr) and compressing the mixture into a thin, transparent disc. This process allows the infrared light of the spectrometer to pass through the sample, which would otherwise be opaque.
The fundamental challenge with solid samples is their opacity to infrared light. KBr pellets solve this by dispersing the solid sample within a potassium bromide (KBr) matrix, which is transparent to IR radiation, effectively turning the sample into a "window" for analysis.

Why KBr is the Standard for Solid Sample Analysis
Understanding why this specific method is so prevalent requires looking at the challenges of solid-state analysis and the unique properties of potassium bromide.
The Challenge of Solid Samples
Most solid materials are opaque and will either block or scatter an infrared beam entirely. This makes it impossible to obtain a useful absorption spectrum, which relies on light passing through the substance to measure which frequencies are absorbed.
The Unique Properties of KBr
Potassium bromide is the ideal medium for this technique for two key reasons. First, it is transparent to infrared radiation across the vast majority of the mid-IR range, meaning it does not have its own absorption bands that would interfere with the sample's spectrum. Second, it is a soft, crystalline salt that flows under pressure, allowing it to form a solid, glass-like, and transparent pellet when compressed.
How the Pellet Enables Measurement
When the finely ground sample is homogeneously dispersed within the KBr matrix, the individual sample particles become suspended. The IR beam passes through the transparent KBr, and its energy is absorbed only by the chemical bonds within the sample particles. The detector then records which frequencies were absorbed, generating the sample's unique spectral fingerprint.
Key Factors for a High-Quality Spectrum
The quality of your KBr pellet directly determines the quality of your results. A poorly made pellet will produce a noisy and uninterpretable spectrum.
The Critical Role of Concentration
The concentration of your sample in the KBr is paramount. The ideal range is typically 0.2% to 1% by weight.
Because a solid pellet is much thicker than a typical liquid sample film, a very low concentration is required. Too little sample results in weak, barely detectable peaks. Too much sample causes the IR beam to be completely absorbed or scattered, resulting in "flat-lined" peaks and a very noisy spectrum.
The Importance of Homogeneity
The sample must be ground into a fine powder and mixed homogeneously with the KBr powder. This ensures the sample is evenly distributed throughout the pellet.
An uneven mixture leads to inconsistent absorption and scattering of the IR light as it passes through different parts of the pellet, distorting the spectrum and making it non-representative of the bulk material.
The Problem of Water Contamination
Potassium bromide is hygroscopic, meaning it readily absorbs moisture from the air. Water has very strong and broad absorption bands in the infrared spectrum.
If your KBr powder, sample, or die set is not perfectly dry, water bands will appear in your spectrum, potentially obscuring important peaks from your actual sample. This is why heating the die set and using dry KBr is critical.
Understanding the Trade-offs and Common Pitfalls
Achieving a clear, transparent pellet is a skill that requires avoiding several common mistakes.
Pitfall 1: Incorrect Particle Size
You must grind the sample, not the KBr powder. The sample particles must be smaller than the wavelength of the IR light to prevent light scattering (known as Mie scattering). Large particles cause a sloping baseline and reduce the clarity of your spectrum.
Pitfall 2: Cloudy or Cracked Pellets
A cloudy or opaque pellet is a clear sign of a problem. This is often caused by insufficient pressure, trapped air, or moisture. It can also occur if the sample concentration is too high, which prevents the KBr from properly coalescing into a clear disc.
Pitfall 3: Non-Uniform Thickness
Using too much KBr/sample mixture can create a pellet that is too thick. An overly thick pellet can be difficult to press into a transparent state and may lead to overly intense absorption bands. A good pellet is made from just enough material to form a thin, even disc.
Making the Right Choice for Your Analysis
The KBr pellet technique is versatile, but your specific goal dictates where to focus your attention.
- If your primary focus is qualitative identification: Your main goal is eliminating contamination. Ensure your KBr is dry and your grinding equipment is clean to prevent spurious peaks from water or other residues.
- If your primary focus is quantitative analysis: You must prioritize a precise sample concentration and a perfectly homogeneous mixture to ensure the spectral intensity is directly and consistently proportional to the amount of sample.
- If you are troubleshooting a noisy or sloping spectrum: First, check your sample's particle size and the pellet's physical clarity, as light scattering is the most common cause of these issues.
Mastering the KBr pellet technique transforms it from a procedural chore into a powerful tool for unlocking the chemical identity of solid materials.
Summary Table:
| Key Factor | Importance for Quality Spectrum |
|---|---|
| Sample Concentration | Critical range: 0.2% to 1% by weight. Too much causes noise; too little yields weak peaks. |
| Homogeneity | Ensures even distribution of sample in KBr for consistent, accurate spectral results. |
| Dry Conditions | Prevents water contamination, which can obscure sample peaks with strong absorption bands. |
| Particle Size | Sample particles must be smaller than IR wavelength to avoid light scattering and baseline issues. |
Unlock precise chemical analysis in your lab with KINTEK's expertise.
Mastering the KBr pellet technique is essential for reliable FTIR spectroscopy results. At KINTEK, we specialize in providing high-quality lab equipment and consumables—including reliable KBr and pellet presses—to help you achieve clear, contamination-free spectra every time.
Whether you're focused on qualitative identification or quantitative analysis, our products are designed to meet the rigorous demands of laboratory professionals. Let us support your research with equipment that ensures accuracy and efficiency.
Contact us today to learn how KINTEK can enhance your solid sample analysis workflow!
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- kbr pellet press 2t
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
People Also Ask
- How does a laboratory hydraulic press improve XRF accuracy for catalyst samples? Enhance Precision & Signal Stability
- What is the pressed powder pellet method? A Guide to Accurate FTIR Sample Preparation
- How hot is a hydraulic press? Understanding the Critical Heat in Your Hydraulic System
- What is the use of KBr? Master Sample Prep for Accurate IR Spectroscopy
- Why use KBr for IR? Achieve Clear, Unobstructed Spectra for Solid Samples



















