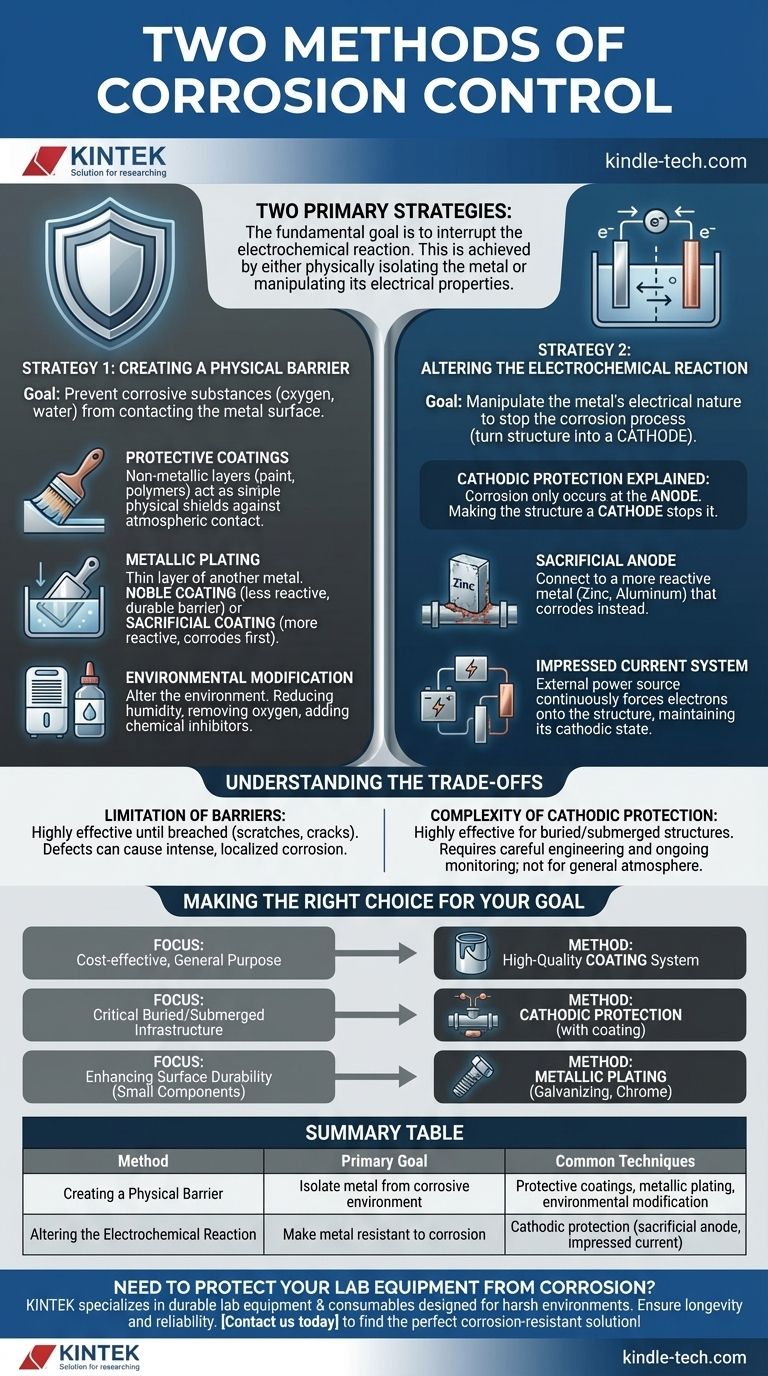
The two primary strategies for controlling corrosion are to either create a barrier that isolates the metal from its corrosive environment or to alter the electrochemical conditions to make the metal inherently resistant to corrosion. Methods like coatings and plating fall into the first category, while techniques such as cathodic protection fall into the second.
The fundamental goal of corrosion control is to interrupt the electrochemical reaction that degrades metal. This is achieved either by physically isolating the metal from its environment or by manipulating the metal's electrical properties to force it to resist the reaction.
Strategy 1: Creating a Physical Barrier
This approach focuses on preventing corrosive substances like oxygen and water from making contact with the metal's surface. It is the most common and intuitive method of corrosion control.
Protective Coatings
Coatings are non-metallic layers, such as paint or polymers, applied to the metal surface.
They act as a simple physical shield, blocking the chemical reaction between the metal and the surrounding atmosphere.
Metallic Plating
Plating involves applying a thin layer of another metal onto the surface.
This can be a noble coating (using a less reactive metal like chromium) to form a durable barrier or a sacrificial coating (using a more reactive metal like zinc in galvanization) that corrodes first, protecting the underlying metal.
Environmental Modification
This involves altering the environment itself to make it less corrosive.
Techniques include reducing humidity, removing dissolved oxygen from water, or adding chemical inhibitors that form a protective film on the metal's surface.
Strategy 2: Altering the Electrochemical Reaction
This advanced strategy doesn't block the environment but instead manipulates the electrical nature of the metal to stop the corrosion process. It is based on the principle that corrosion is an electrochemical cell.
Cathodic Protection Explained
This method turns the entire structure you want to protect into the cathode of an electrochemical cell.
Since corrosion (the loss of metal) only occurs at the anode, making the structure a cathode effectively stops it from corroding.
How it Works
This is achieved in one of two ways. You can connect the structure to a more reactive sacrificial anode (like a block of zinc or aluminum) that corrodes instead.
Alternatively, you can use an impressed current system, which uses an external power source to continuously force electrons onto the structure, maintaining its cathodic state.
Understanding the Trade-offs
No single method is perfect for all applications. Understanding their limitations is critical for effective long-term protection.
The Limitation of Barriers
Coatings and platings are highly effective until they are breached. A scratch, crack, or pinhole can expose the underlying metal.
In some cases, this small defect can create a focal point for intense, localized corrosion that is more damaging than uniform corrosion on an unprotected surface.
The Complexity of Cathodic Protection
Cathodic protection is highly effective for structures buried in soil or submerged in water, such as pipelines and ship hulls.
However, it requires careful engineering design and ongoing monitoring to ensure it is working correctly. It is not a practical solution for preventing general atmospheric corrosion on its own.
Making the Right Choice for Your Goal
Selecting the correct method depends entirely on the component, its environment, and the project's budget and lifespan requirements.
- If your primary focus is cost-effective, general-purpose protection: A high-quality coating system like industrial paint is the standard choice.
- If your primary focus is protecting critical buried or submerged infrastructure: Cathodic protection, almost always used in combination with a heavy-duty coating, is the essential solution.
- If your primary focus is enhancing surface durability for small components: Metallic plating, such as galvanizing or chrome plating, offers excellent and robust protection.
Understanding these core strategies allows you to select the most effective and efficient method to preserve the integrity of any metallic structure.
Summary Table:
| Method | Primary Goal | Common Techniques |
|---|---|---|
| Creating a Physical Barrier | Isolate metal from corrosive environment | Protective coatings, metallic plating, environmental modification |
| Altering the Electrochemical Reaction | Make metal resistant to corrosion | Cathodic protection (sacrificial anode, impressed current) |
Need to protect your lab equipment from corrosion? KINTEK specializes in providing durable lab equipment and consumables designed to withstand harsh environments. Our solutions ensure the longevity and reliability of your laboratory assets. Contact us today to find the perfect corrosion-resistant equipment for your needs!
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- Flat Corrosion Electrolytic Electrochemical Cell
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Anti-Cracking Press Mold for Lab Use
People Also Ask
- How does a gold sputter coater work? A Step-by-Step Guide to Creating Conductive Coatings
- How does a hot air furnace work? Understanding Safe and Efficient Home Heating
- What does sputtering mean in business? A Strategic Manufacturing Process for Competitive Advantage
- What is the effect of sintering temperature? Master the Key to Material Density and Strength
- How do baffled flasks and orbital shaker incubators facilitate yeast screening? Optimize Oxygen for Lipid Production
- What are the factors affecting the heat treatment of steel? Master the Process for Superior Material Properties
- At what temperature is conventional pyrolysis done? Unlock the Right Temperature for Your Desired Product
- What makes Ultra-Low Temperature freezers energy efficient? Key Design & Operational Strategies



















