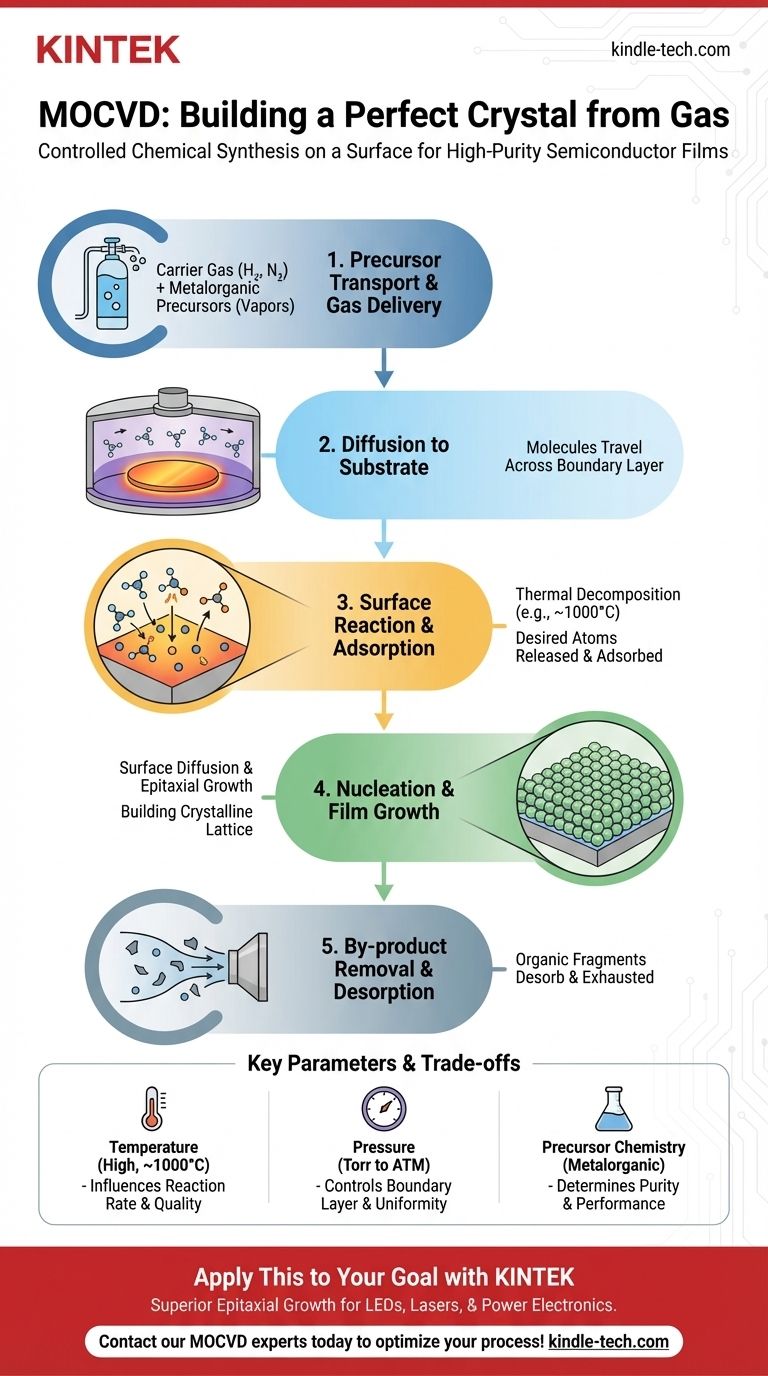
At its core, the MOCVD process involves introducing precise, volatile chemical vapors into a reaction chamber where they decompose on a heated surface to form a highly pure, crystalline thin film. This process can be broken down into five fundamental stages: precursor transport, diffusion to the substrate, surface reaction, film growth, and by-product removal. Each step is meticulously controlled to build the final material one atomic layer at a time.
MOCVD is not merely a deposition technique; it is a controlled chemical synthesis on a surface. The central challenge is managing a delicate balance of gas flow, temperature, and pressure to ensure that chemical reactions happen exclusively on the substrate, resulting in a perfect crystalline structure.

The Goal: Building a Perfect Crystal from Gas
Before detailing the steps, it's crucial to understand the objective. Metalorganic Chemical Vapor Deposition (MOCVD) is a sophisticated form of Chemical Vapor Deposition (CVD) used to create extremely high-quality semiconductor films.
What Makes MOCVD Special?
The "MO" in MOCVD stands for metalorganic. This refers to the precursor chemicals used, which are organic compounds containing metal atoms.
These precursors are designed to be volatile at low temperatures but to break apart (decompose) predictably at high temperatures, releasing their metal atoms onto a surface.
The Core Principle: Controlled Decomposition
The entire process is engineered to create a reaction zone limited to the heated surface of a wafer, known as the substrate.
By precisely controlling the environment, we can ensure that atoms land on the substrate and arrange themselves into a perfect crystal lattice, a process called epitaxial growth.
A Step-by-Step Breakdown of the MOCVD Process
Each stage of the MOCVD process is a distinct physical and chemical event that builds upon the last. The entire sequence takes place inside a highly controlled system containing a gas delivery system, a reaction chamber, a heating source, and an exhaust system.
Step 1: Precursor Transport and Gas Delivery
The process begins by feeding the chosen precursor chemicals into the reactor. These metalorganic compounds are often liquid or solid at room temperature.
A carrier gas (like hydrogen or nitrogen) is bubbled through the liquid precursors to pick up their vapor and carry them in precise concentrations.
These reactive gases are then mixed and delivered into the reaction chamber through a carefully designed gas delivery system. The accuracy of this mixing determines the composition of the final material.
Step 2: Diffusion to the Substrate Surface
Inside the reactor, the gas mixture flows over the heated substrate. However, the gas directly touching the hot surface doesn't move, creating a static "boundary layer."
The reactive precursor molecules must travel from the main gas flow across this boundary layer to reach the substrate. This journey is driven by diffusion.
Step 3: Adsorption and Surface Reaction
Once a precursor molecule reaches the hot substrate, it "sticks" to the surface in a process called adsorption.
The intense heat of the substrate provides the energy needed to break the chemical bonds within the precursor molecule. This thermal decomposition releases the desired atoms (e.g., gallium, arsenic) onto the surface.
Step 4: Nucleation and Film Growth
The freed atoms are now adsorbed on the surface and can move around via surface diffusion.
These atoms migrate to energetically favorable locations, finding their place within the crystal lattice of the substrate. This initiates the growth of a new atomic layer.
As this process repeats, the film grows layer by layer, replicating the crystalline structure of the substrate below.
Step 5: Desorption and By-product Removal
The chemical reaction leaves behind unwanted molecular fragments, known as by-products (for example, the organic parts of the original precursor).
These by-products must detach from the surface (desorption) and be carried away by the gas flow. Efficient removal is critical to prevent them from being incorporated as impurities in the growing film.
Understanding the Key Parameters and Trade-offs
The success of MOCVD hinges on a precise balance of several interdependent variables. Mismanaging any one of them can compromise the quality of the final film.
The Critical Role of Temperature
Temperature is the primary engine of the MOCVD reaction. It must be high enough to efficiently decompose the precursors on the surface.
However, if the temperature is too high, precursors can react in the gas phase before even reaching the substrate, leading to particle formation and defects in the film. Typical process temperatures are very high, often around 1000°C.
The Influence of Pressure
The reactor's pressure, ranging from a few torr to atmospheric pressure, directly impacts gas flow dynamics and the thickness of the boundary layer.
Lower pressures can lead to more uniform deposition but may also change the chemical reaction pathways. The chosen pressure is a critical parameter for controlling growth rate and film quality.
Precursor Chemistry is Everything
The selection of the metalorganic precursor is paramount. An ideal precursor is stable, non-toxic, sufficiently volatile, and decomposes cleanly at the desired temperature, leaving behind only the desired atoms.
The chemistry of the precursor directly influences the purity, growth rate, and ultimate performance of the semiconductor device.
Applying This to Your Goal
The complexity of MOCVD is justified by the unparalleled quality of the materials it can produce. The reason to choose it depends on your specific objective.
- If your primary focus is the highest crystalline quality: MOCVD is the industry standard for creating the near-perfect epitaxial films required for high-performance lasers, LEDs, and power electronics.
- If your primary focus is creating complex compound semiconductors: The precise gas-phase mixing in MOCVD allows for the creation of ternary (e.g., InGaAs) or quaternary (e.g., AlInGaN) alloys with exact, repeatable compositions.
- If your primary focus is scalable, high-volume manufacturing: Modern MOCVD reactors are highly automated systems capable of processing large-diameter wafers, making them the workhorse of the global optoelectronics industry.
By orchestrating this sequence of chemical and physical events, MOCVD transforms simple gases into some of the most advanced materials on Earth.
Summary Table:
| Step | Process | Key Action |
|---|---|---|
| 1 | Precursor Transport | Vaporized metalorganic compounds carried by carrier gas into reactor |
| 2 | Diffusion to Substrate | Molecules travel across boundary layer to heated wafer surface |
| 3 | Surface Reaction | Thermal decomposition releases desired atoms onto substrate |
| 4 | Film Growth | Atoms incorporate into crystal lattice via epitaxial growth |
| 5 | By-product Removal | Organic fragments desorb and are carried away by gas flow |
Ready to achieve superior epitaxial film growth? KINTEK specializes in advanced MOCVD systems and lab equipment for semiconductor research and production. Our expertise in temperature control, gas delivery, and reactor design ensures you get the highest quality films for your LEDs, lasers, and power electronics.
Contact our MOCVD experts today to discuss how we can optimize your thin-film deposition process!
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- What is the function of heating tapes in CVD? Ensure Vapor Phase Stability & Prevent Line Blockage
- What is the DC sputtering method? A Guide to Thin Film Deposition for Conductive Coatings
- What is Vapour deposition process? A Guide to PVD & CVD Thin-Film Coating Methods
- What are the disadvantages of sputtering? Key Challenges and Trade-offs for Thin-Film Deposition
- Why is the removal of byproducts crucial in a CVD process? Ensure Film Purity and High Semiconductor Yields
- What is deposition in semiconductor process? Building the Atomic Layers of Modern Chips
- What is the difference between selective laser sintering and electron beam melting? Sintering vs. Melting for Additive Manufacturing
- How thick is CVD coating? Optimize Your Tool's Wear Resistance & Durability