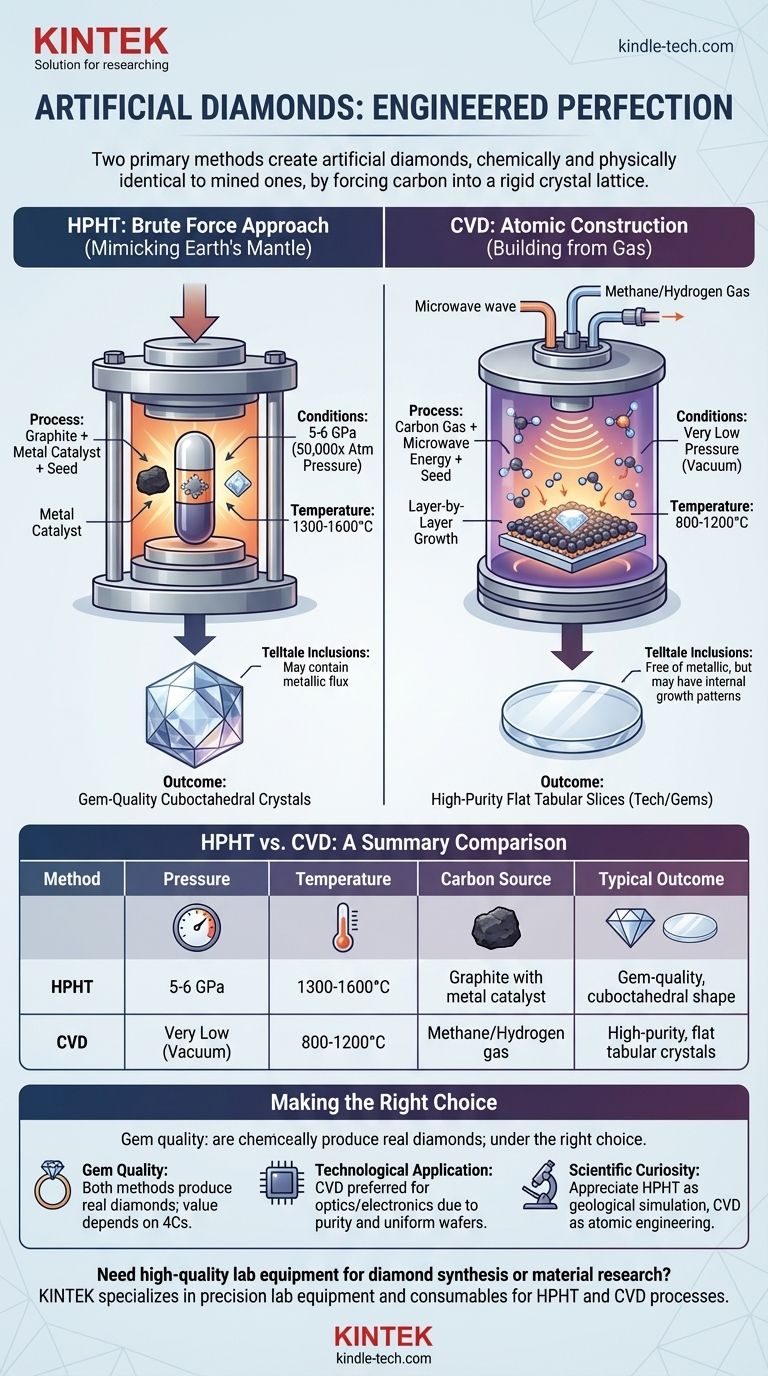
To create an artificial diamond, you must use one of two primary methods, each involving drastically different conditions. The first, High Pressure/High Temperature (HPHT), replicates the brutal force of the Earth's mantle. The second, Chemical Vapor Deposition (CVD), "grows" a diamond atom-by-atom from a superheated gas. Both methods produce a final product that is chemically, physically, and optically identical to a mined diamond.
The core challenge in creating a diamond is forcing carbon atoms into a highly stable, rigid crystal lattice. You can either achieve this with immense physical force (HPHT) or by meticulously engineering the atomic environment (CVD).

The Brute Force Approach: High Pressure/High Temperature (HPHT)
The HPHT method is the original technique for creating diamonds and directly mimics the conditions deep within the Earth where natural diamonds form.
Replicating the Earth's Mantle
The goal of HPHT is to create an environment where carbon's most stable form is diamond, not graphite (the form found in pencils). This requires simulating the conditions of the Earth's upper mantle.
The Key Ingredients
A process starts with a source of pure carbon, like graphite. This carbon is placed in a capsule with a metal catalyst (such as iron, nickel, or cobalt) and a tiny diamond "seed" crystal.
The Required Conditions
The capsule is subjected to immense pressure of 5 to 6 gigapascals (GPa), which is over 50,000 times the atmospheric pressure at sea level. Simultaneously, it is heated to temperatures between 1300–1600°C (2372–2912°F).
The Outcome: Gem-Quality Crystals
Under this extreme heat and pressure, the metal catalyst dissolves the carbon source. The carbon atoms then migrate through the molten metal and precipitate onto the cooler diamond seed, crystallizing into a new, larger diamond. The process can take several days to weeks.
The Atomic Construction Approach: Chemical Vapor Deposition (CVD)
CVD is a newer technique that builds a diamond from the ground up, more akin to 3D printing on an atomic scale. It does not rely on high pressure.
Building from the Atom Up
Instead of forcing a solid carbon source into a diamond, CVD starts with a carbon-containing gas. The method deposits carbon atoms one by one onto a substrate to grow a diamond crystal in layers.
The Key Ingredients
This process begins with a thin slice of a diamond seed crystal placed inside a vacuum chamber. The chamber is then filled with a carbon-rich gas, typically methane, along with other gases like hydrogen.
The Required Conditions
The chamber is heated to high temperatures of 800–1200°C (1472–2192°F), but at very low pressure—essentially a vacuum. Energy, usually from microwaves, is introduced into the chamber to break the gas molecules apart, liberating the carbon atoms.
The Outcome: High-Purity Slices
These freed carbon atoms then settle onto the diamond seed plate, growing the crystal layer by layer. The result is often a flat, tabular diamond crystal of very high purity. This process is highly controlled and can produce large diamonds suitable for both gems and advanced technology.
Understanding the Trade-offs and Differences
While both methods produce real diamonds, the conditions under which they are made leave subtle clues that a gemologist can identify.
HPHT vs. CVD: A Matter of Growth
HPHT diamonds grow in a cuboctahedral shape, mirroring their natural counterparts. In contrast, CVD diamonds grow in flat layers, resulting in a tabular crystal structure before cutting.
Telltale Inclusions
The creation process can leave behind microscopic identifying marks. HPHT diamonds may contain tiny inclusions of the metallic flux used during their growth. CVD diamonds, on the other hand, are free of metallic inclusions but may exhibit unique internal growth patterns or dark pinpoint carbon spots.
Color and Treatment
Initially, HPHT diamonds were often yellowish or brownish due to nitrogen in the growth environment, while CVD diamonds could have a brownish hue from other factors. However, post-growth treatment processes (often involving heat or irradiation) can permanently remove this coloration, making the final gems colorless.
Making the Right Choice for Your Goal
Understanding the creation conditions helps you appreciate the final product, whether it's for jewelry, science, or industry.
- If your primary focus is gem quality: Know that both methods produce real diamonds. The final quality and value are determined by the 4Cs (cut, color, clarity, and carat), not the growth method.
- If your primary focus is technological application: CVD is often preferred for optics and electronics, as it allows for the creation of large, uniform, high-purity diamond wafers with specific properties.
- If your primary focus is scientific curiosity: Appreciate HPHT as a triumph of geological simulation and CVD as a masterpiece of atomic-scale engineering.
Ultimately, both methods demonstrate that the extreme conditions of nature can be successfully replicated and even refined through human ingenuity.
Summary Table:
| Method | Pressure | Temperature | Carbon Source | Typical Outcome |
|---|---|---|---|---|
| HPHT | 5-6 GPa (50,000x atmospheric) | 1300–1600°C | Graphite with metal catalyst | Gem-quality crystals, cuboctahedral shape |
| CVD | Very low (vacuum) | 800–1200°C | Methane/Hydrogen gas | High-purity, flat tabular crystals for tech/gems |
Need high-quality lab equipment for diamond synthesis or material research? KINTEK specializes in precision lab equipment and consumables for advanced processes like HPHT and CVD. Whether you're growing diamonds for gemological, scientific, or industrial applications, our solutions ensure reliable performance and exacting standards. Contact us today to discuss how we can support your laboratory's unique needs!
Visual Guide
Related Products
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- CVD Diamond Domes for Industrial and Scientific Applications
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
People Also Ask
- What is Vapour Phase Epitaxy (VPE)? Master High-Purity Semiconductor Growth for Electronics
- What is the process of thin film deposition? A Guide to PVD, CVD, and Coating Techniques
- What are the steps involved in thin film deposition? Master the 5 Core Stages for Precision Coatings
- What is the vapor deposition growth process? Grow High-Performance Thin Films Atom by Atom
- What is the temperature range for chemical vapor deposition? From 100°C to 1200°C for Perfect Thin Films
- What is the pressure for sputtering? Optimize Your Thin Film Density and Coverage
- What are the components of a CVD reactor? A Guide to the Core Systems for Thin Film Deposition
- What is the basic atomic layer deposition? A Guide to Ultra-Thin Film Precision



















