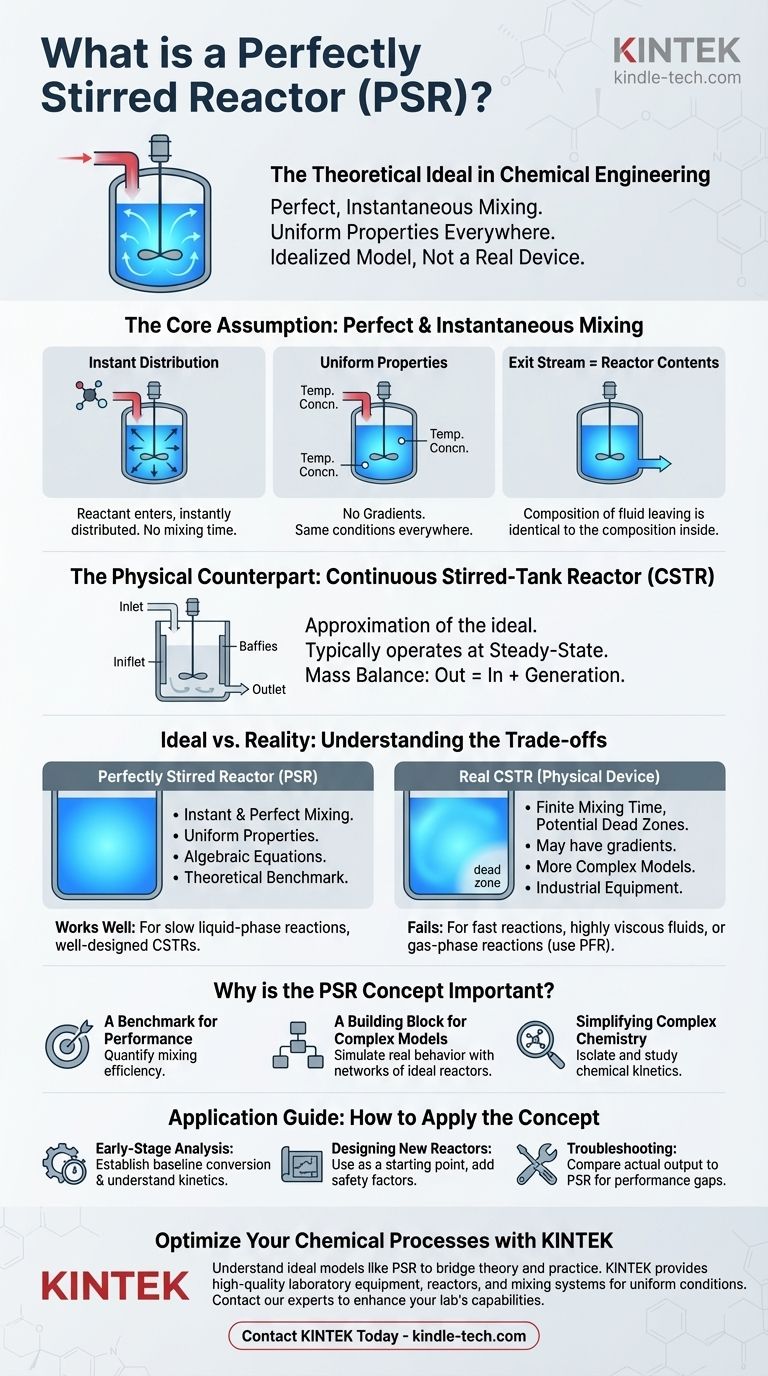
In chemical engineering, a Perfectly Stirred Reactor (PSR) is an idealized reactor model where perfect, instantaneous mixing occurs. This means that any fluid entering the reactor is immediately dispersed, resulting in completely uniform properties—such as temperature and chemical concentration—at every single point within the reactor volume. It is the theoretical basis for the Continuous Stirred-Tank Reactor (CSTR).
The Perfectly Stirred Reactor is not a real-world device but a powerful mathematical abstraction. Its value lies in simplifying complex reaction analysis, providing a crucial benchmark against which the performance of real industrial reactors is measured and improved.

The Core Assumption: Perfect and Instantaneous Mixing
The entire concept of the PSR hinges on one powerful, simplifying assumption: mixing is infinitely fast. This has several critical consequences for how we model chemical reactions.
What "Perfectly Stirred" Really Means
At the moment a reactant molecule enters the reactor, it is assumed to be instantly distributed throughout the entire volume. There is no "entry zone" or "mixing time" to consider.
Uniform Properties Everywhere
Because of this perfect dispersion, there are no gradients inside the reactor. The temperature, pressure, and concentration of each chemical species are identical whether you measure them near the inlet, at the wall, or in the center.
The Exit Stream is the Reactor Itself
A key outcome of this uniformity is that the composition of the fluid leaving the reactor is exactly the same as the composition of the fluid inside the reactor. This is the most important characteristic for mathematical modeling.
The CSTR: The Physical Counterpart
The "Perfectly Stirred Reactor" is the ideal model, while the Continuous Stirred-Tank Reactor (CSTR) is the physical piece of equipment that engineers design to approximate this ideal.
Steady-State Operation
CSTRs are typically operated at steady-state. This means the rate of mass flowing into the reactor is equal to the rate of mass flowing out, and the conditions (temperature, concentration) inside the reactor do not change over time.
The Governing Principle
The model is governed by a simple mass balance: Accumulation = In - Out + Generation. For a CSTR at steady-state, Accumulation is zero, so the equation simplifies to Out = In + Generation. This turns complex differential equations into more manageable algebraic equations, making design calculations vastly simpler.
Understanding the Trade-offs: Ideal Model vs. Reality
The PSR is a tool, and like any tool, it has limitations. Trusting the model requires understanding where it deviates from the real world.
The Limitation of Mixing Time
In any real tank, mixing is not instantaneous. It takes a finite amount of time for an impeller to circulate the fluid. This can create "dead zones" (areas of low mixing) or "short-circuiting" (where fluid bypasses the tank and exits too quickly).
When the Model Works Well
The PSR/CSTR model is highly effective for many liquid-phase reactions, especially those that are relatively slow compared to the mixing rate. In a well-designed CSTR with proper baffling and agitation, the contents can be nearly uniform, making the model a very accurate approximation.
When the Model Fails
This model is a poor choice for systems where mixing is slow or reactions are extremely fast. This includes highly viscous fluids, laminar flow conditions, or gas-phase reactions like combustion, which are often better described by a Plug Flow Reactor (PFR) model.
Why This Idealized Model is So Important
Despite its limitations, the PSR concept is a cornerstone of chemical reaction engineering for several fundamental reasons.
A Benchmark for Performance
The ideal PSR provides a theoretical maximum for conversion under mixed conditions. By comparing a real reactor's output to the PSR model's prediction, engineers can quantify mixing efficiency and diagnose operational problems.
A Building Block for Complex Models
No real reactor is perfectly mixed. However, complex real-world systems can be effectively modeled as a network of ideal reactors. For example, a poorly performing CSTR might be modeled as a small, ideal CSTR connected to a "dead zone" and a "bypass stream" to accurately capture its behavior.
Simplifying Complex Chemistry
The primary power of the PSR is its mathematical simplicity. By assuming uniform properties, it allows engineers to isolate and study chemical kinetics without the complicating factor of physical transport phenomena like diffusion and convection.
How to Apply the PSR Concept
Your application of the PSR/CSTR model depends entirely on your goal.
- If your primary focus is early-stage reaction analysis: Use the PSR model to quickly establish baseline conversion rates and understand the fundamental kinetics of your chemical system.
- If your primary focus is designing a new physical reactor: Use the CSTR equations as the starting point for sizing and initial design, but build in safety factors to account for real-world mixing inefficiencies.
- If your primary focus is troubleshooting an existing reactor: Compare your reactor's actual output against the ideal PSR model's predictions to identify and quantify performance gaps caused by poor mixing.
Mastering the concept of the perfectly stirred reactor is not about finding a flawless machine, but about wielding a powerful framework for analyzing and designing real-world chemical processes.
Summary Table:
| Aspect | Perfectly Stirred Reactor (PSR) | Real Continuous Stirred-Tank Reactor (CSTR) |
|---|---|---|
| Mixing | Instantaneous and perfect | Finite mixing time, potential dead zones |
| Internal Properties | Uniform temperature and concentration everywhere | May have gradients |
| Exit Stream | Identical to reactor contents | May differ slightly due to imperfect mixing |
| Mathematical Model | Algebraic equations (steady-state) | More complex, may require safety factors |
| Primary Use | Theoretical benchmark and kinetic analysis | Physical equipment for industrial processes |
Optimize Your Chemical Processes with KINTEK
Whether you are designing a new reactor, scaling up a process, or troubleshooting an existing system, understanding ideal models like the PSR is crucial. At KINTEK, we specialize in providing high-quality laboratory equipment and consumables that help you bridge the gap between theory and practice.
Our reactors and mixing systems are designed to help you achieve the most uniform conditions possible, getting you closer to the ideal model for accurate and efficient results.
Let KINTEK be your partner in precision. Contact our experts today to discuss how our solutions can enhance your laboratory's capabilities and drive your research forward.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
People Also Ask
- What is the primary function of fluidized bed or conical reactors in biomass fast pyrolysis? Maximize Bio-oil Yield
- What technical advantages does a high-pressure sealed reactor offer for transesterification? Boost Biodiesel Efficiency
- What is the difference between a reactor and a reaction vessel? Understanding the Core Component vs. the Complete System
- What is the purpose of purging a high-pressure reactor with nitrogen? Ensure High-Yield Hydrothermal Liquefaction
- What is the primary function of a high-pressure reactor in APTES-modified TiO2 prep? Enhance Synthesis Efficiency
- What is the primary function of a CSTR in the Cu-Cl cycle? Optimize Oxygen Production at High Temperatures
- Why is a PTFE-lined high-pressure autoclave required for hydrothermal doping? Achieve Pure Graphene Synthesis
- What is the advantage of using high-pressure hydrothermal reactors to treat biomass waste? Efficient Resource Recovery



















