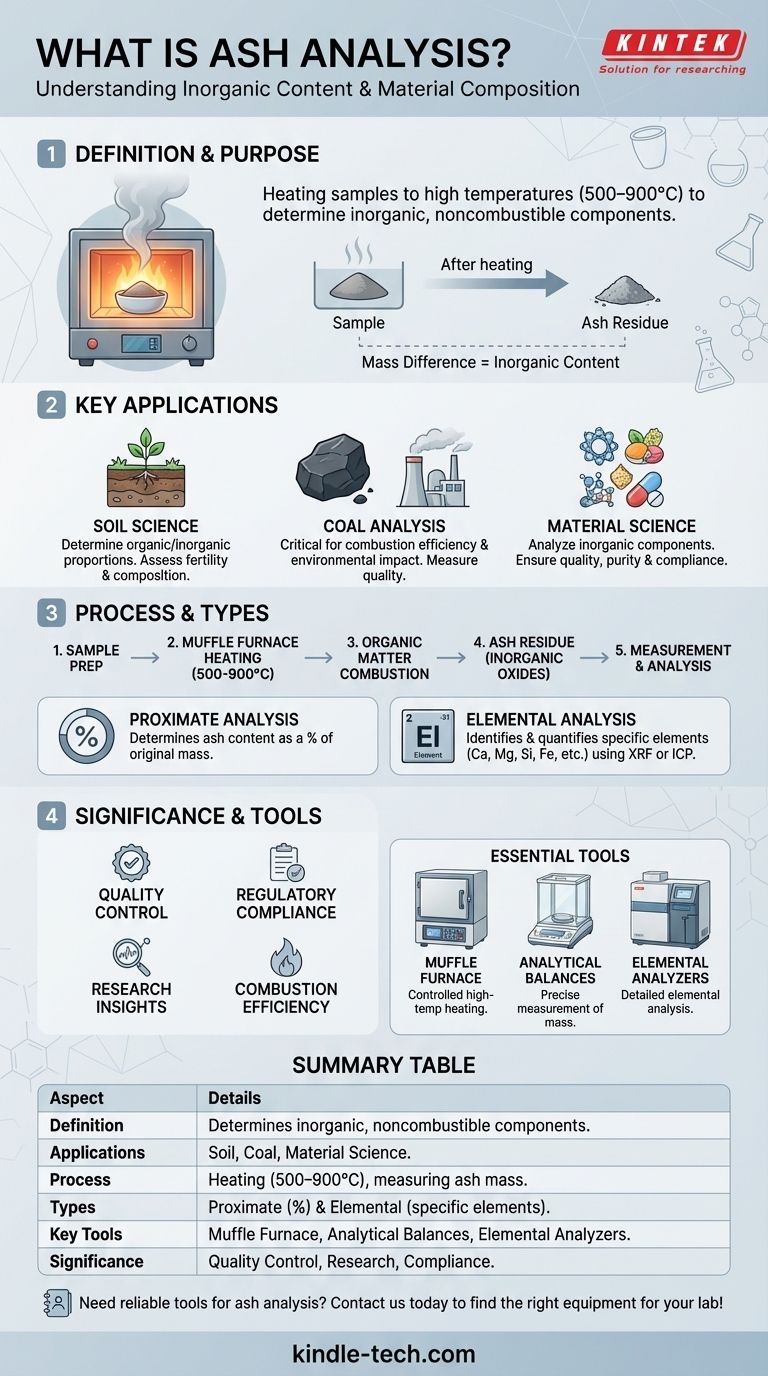
Ash analysis is a process used in analytical chemistry to determine the inorganic, noncombustible components of a material by heating it to high temperatures, leaving behind ash residue. This residue is then analyzed to understand the elemental composition of the original sample. Ash analysis is particularly useful in fields like soil science, coal analysis, and material science, where it helps quantify the inorganic content and assess the quality or composition of a material. The process involves comparing the mass of the sample before and after ashing to calculate the ash content, which is a critical parameter in various industries.

Key Points Explained:
-
Definition and Purpose of Ash Analysis
- Ash analysis is a technique used to determine the inorganic, noncombustible components of a material.
- It involves heating a sample to high temperatures until all organic matter is burned off, leaving behind ash residue.
- The primary purpose is to analyze the elemental composition of the ash, which reflects the inorganic content of the original sample.
-
Applications of Ash Analysis
- Soil Science: Used to determine the organic and inorganic proportions of soil by comparing the mass before and after ashing. This helps in understanding soil fertility and composition.
- Coal Analysis: Ash analysis is critical in assessing coal quality. The ash content of coal is an important parameter for its combustion efficiency and environmental impact.
- Material Science: Used to analyze the inorganic components of materials like polymers, food products, and pharmaceuticals to ensure quality and compliance with standards.
-
Process of Ash Analysis
- The sample is heated in a furnace at high temperatures (typically 500–900°C) until all organic matter is combusted.
- The remaining ash consists of inorganic oxides and other noncombustible materials.
- The mass of the ash is measured and compared to the original sample mass to calculate the ash content.
-
Types of Ash Analysis
- Proximate Analysis: Determines the ash content as a percentage of the original sample mass.
- Elemental Analysis: Identifies and quantifies the specific elements present in the ash, such as calcium, magnesium, silicon, and iron.
-
Significance of Ash Content
- In coal, high ash content reduces combustion efficiency and increases waste generation.
- In soil, ash content provides insights into the mineral composition and organic matter content.
- In food and pharmaceuticals, ash content is a quality control measure to ensure purity and compliance with regulatory standards.
-
Challenges and Considerations
- The temperature and duration of ashing must be carefully controlled to avoid volatilization of certain inorganic compounds.
- Contamination from the furnace or environment can affect the accuracy of the results.
- The choice of ashing method (dry ashing or wet ashing) depends on the sample type and the elements being analyzed.
-
Tools and Equipment for Ash Analysis
- Muffle Furnace: Used for dry ashing, it provides controlled high-temperature heating.
- Analytical Balances: For precise measurement of sample mass before and after ashing.
- Elemental Analyzers: Instruments like X-ray fluorescence (XRF) or inductively coupled plasma (ICP) for detailed elemental analysis of the ash.
-
Interpretation of Results
- The ash content is expressed as a percentage of the original sample mass.
- Elemental analysis results are used to identify the specific inorganic components and their concentrations.
- These results are critical for quality control, research, and regulatory compliance in various industries.
By understanding ash analysis, purchasers of equipment and consumables can make informed decisions about the tools and methods needed for accurate and reliable analysis in their specific applications.
Summary Table:
| Aspect | Details |
|---|---|
| Definition | Determines inorganic, noncombustible components of a material. |
| Applications | Soil science, coal analysis, material science. |
| Process | Heating samples to 500–900°C, measuring ash residue mass. |
| Types of Analysis | Proximate (ash content %) and elemental (specific elements). |
| Key Tools | Muffle furnace, analytical balances, elemental analyzers (XRF, ICP). |
| Significance | Quality control, research, and regulatory compliance. |
Need reliable tools for ash analysis? Contact us today to find the right equipment for your lab!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the conditions for a muffle furnace? Ensure Safety, Performance, and Longevity
- How is the ash content determined in a muffle furnace? Master the Gravimetric Analysis Method
- What is done by ashing in muffle furnace? A Guide to Precise Inorganic Content Analysis
- What are the different types of laboratory furnaces? Find the Perfect Fit for Your Application
- How does the calcination process work? Master Thermal Decomposition for Material Purification



















