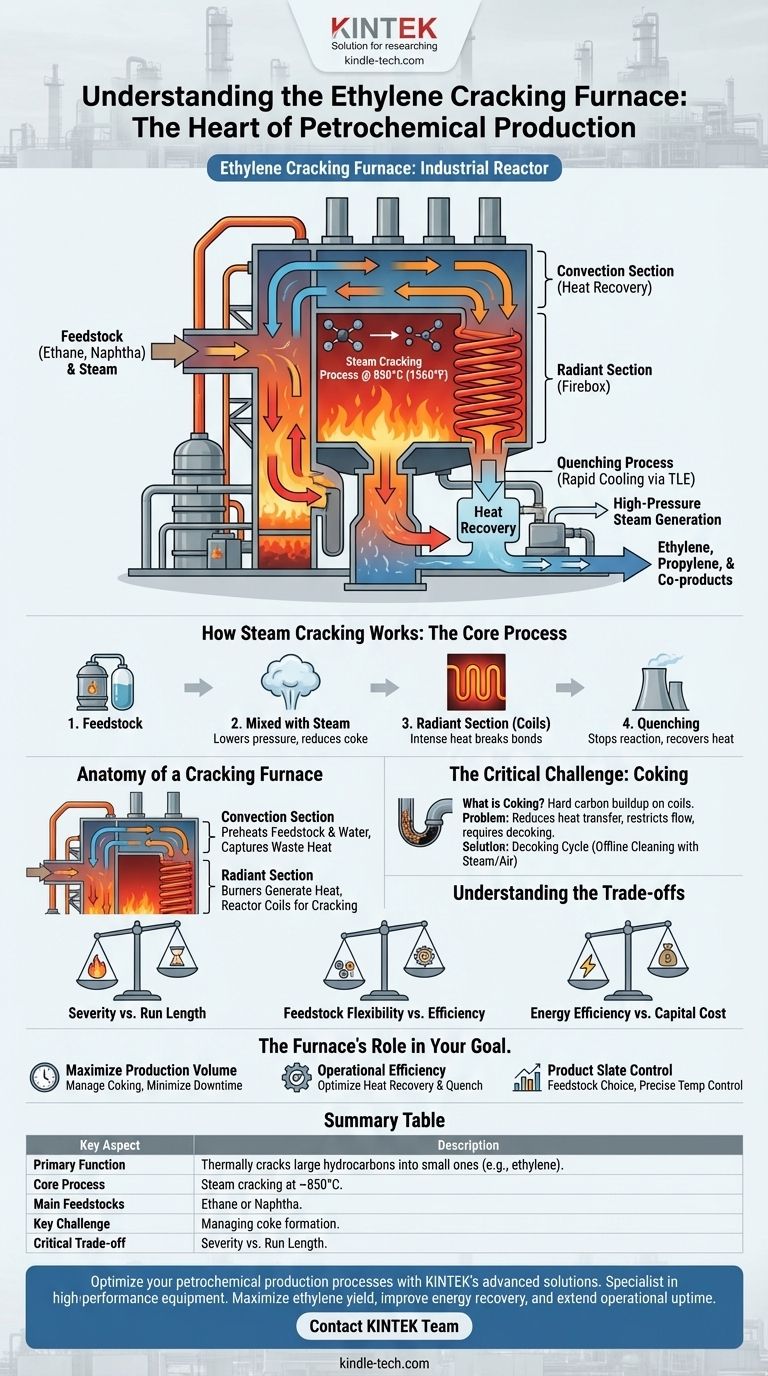
At its core, an ethylene cracking furnace is a massive industrial reactor designed for a single, critical purpose: to thermally break down large hydrocarbon molecules into smaller, more valuable ones. Through a high-temperature process called steam cracking, it transforms feedstocks like ethane or naphtha into ethylene, the primary building block for most of the world's plastics, and other valuable co-products.
The cracking furnace is the high-temperature heart of the modern petrochemical industry. It doesn't just heat a substance; it uses precisely controlled thermal energy to decompose raw materials into the fundamental chemical ingredients required for countless products.

How Steam Cracking Works: The Core Process
The furnace operates on the principle of thermal decomposition. By subjecting hydrocarbons to extreme heat in a controlled environment, the chemical bonds holding them together are broken, or "cracked," resulting in a mixture of smaller molecules.
The Feedstock
The process begins with a hydrocarbon feedstock. This is typically a light hydrocarbon such as ethane (a component of natural gas) or a heavier liquid stream like naphtha (a refinery product). The choice of feedstock dictates the design of the furnace and the mix of products it will yield.
The Role of Steam
The hydrocarbon feedstock is mixed with steam before entering the furnace. The steam serves two critical functions: it lowers the partial pressure of the hydrocarbons, which promotes the desired cracking reactions, and it reduces the formation of coke, a hard carbon byproduct that fouls the equipment.
The Furnace Coils (Radiant Section)
This mixture flows into a network of tubes, or coils, located in the hottest part of the furnace—the radiant section or "firebox." Here, burners heat the coils to temperatures around 850°C (1560°F). In the fraction of a second the mixture spends inside these coils, the intense heat breaks the hydrocarbon molecules apart.
The Quenching Process
The reaction must be stopped almost instantly to lock in the desired product mix and prevent further, unwanted reactions. The hot gas exiting the coils is rapidly cooled in a device called a Transfer Line Exchanger (TLE) or quench cooler. This process also recovers a vast amount of heat, which is used to generate valuable high-pressure steam, significantly improving the plant's overall energy efficiency.
Anatomy of a Cracking Furnace
A cracking furnace is a complex and highly integrated piece of engineering, typically divided into two main sections.
The Convection Section
This is the upper, cooler section of the furnace. Its primary job is heat recovery. Hot flue gases from the burners below travel up through this section, preheating the incoming feedstock, steam, and boiler water. This captures waste heat and dramatically reduces the furnace's fuel consumption.
The Radiant Section (Firebox)
This is the lower, high-temperature heart of the furnace. It houses the burners that generate the immense heat and the reactor coils where the actual cracking reaction takes place. The design of this section is critical for ensuring uniform heat distribution and achieving the desired reaction conditions.
The Critical Challenge: Coking
The single biggest operational challenge in a steam cracker is the management of an unavoidable byproduct: coke.
What is Coking?
Coke is a hard, solid form of carbon that gradually deposits on the inner surface of the reactor coils. It is a natural result of the high-temperature cracking reactions.
Why Coking is a Problem
As coke builds up, it acts as an insulator, reducing heat transfer to the process gas. This forces operators to increase the firing rate to maintain the required temperature. The coke layer also restricts the flow path, increasing the pressure drop across the coils and ultimately limiting the furnace's throughput.
The Decoking Cycle
Eventually, the coke buildup becomes so severe that the furnace must be taken offline for cleaning. This process, known as decoking, involves shutting off the hydrocarbon feed and using a mixture of steam and air to carefully burn the coke out of the coils. This downtime represents a significant loss of production.
Understanding the Trade-offs
Operating a cracking furnace effectively requires balancing several competing factors.
Severity vs. Run Length
Operating at higher temperatures (higher "severity") can increase the yield of valuable ethylene. However, it also dramatically accelerates the rate of coke formation, leading to shorter run times between decoking cycles.
Feedstock Flexibility vs. Efficiency
A furnace designed specifically for a light feedstock like ethane will be highly efficient for that feed but may perform poorly with a heavier feed like naphtha. A more flexible design can handle multiple feedstocks but may not be perfectly optimized for any single one.
Energy Efficiency vs. Capital Cost
Incorporating more extensive heat recovery systems in the convection section increases the furnace's energy efficiency and lowers operating costs. However, these complex systems also significantly increase the initial capital investment required to build the furnace.
The Furnace's Role in Your Goal
Understanding the furnace's function is key to understanding the entire petrochemical value chain. Its performance directly dictates the profitability and efficiency of the plant.
- If your primary focus is maximizing production volume: The critical goal is managing the coking rate to extend the "on-stream" time and minimize downtime for decoking.
- If your primary focus is operational efficiency: The design of the heat recovery systems in the convection section and the quench exchangers is paramount to minimizing fuel consumption.
- If your primary focus is product slate control: The choice of feedstock and the precise control of furnace operating temperatures are the primary levers for determining the final yield of ethylene, propylene, and other co-products.
Ultimately, the ethylene cracking furnace is the powerful engine that converts raw fossil fuels into the high-value chemical building blocks that form the basis of our modern material world.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Function | Thermally cracks large hydrocarbon molecules into smaller ones (e.g., ethylene). |
| Core Process | Steam cracking at temperatures around 850°C (1560°F). |
| Main Feedstocks | Ethane (from natural gas) or naphtha (from refineries). |
| Key Challenge | Managing coke formation, which requires periodic decoking cycles. |
| Critical Trade-off | Higher severity (temperature) increases ethylene yield but shortens run length. |
Optimize your petrochemical production processes with KINTEK's advanced solutions. As a specialist in high-performance laboratory and industrial equipment, we understand the critical balance between furnace severity, efficiency, and run length. Whether your goal is maximizing ethylene yield, improving energy recovery, or extending operational uptime, our expertise can help you achieve it. Contact our team today to discuss how we can support your specific needs in catalysis research, materials testing, and process optimization.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vertical Laboratory Tube Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the catalysts for catalytic pyrolysis? Unlock the Key to Optimizing Biofuel and Chemical Yields
- What are the environmental issues with biomass? The Hidden Costs of a 'Green' Energy Source
- Purpose of Constant Temperature Drying for TiZrN Coatings? Ensure Perfect Laser Carburizing Results
- Is pyrolysis oil flammable? Understanding Its Combustible Nature and Critical Safety Risks
- What are the uses of biochar from pyrolysis? Engineer Its Properties for Your Specific Goal
- Why argon is the usual gas for the sputtering processes? Optimize Your Thin Film Deposition
- What are the applications of IR spectrometry? Identify Chemical Structures for Quality Control and Research
- What are the 3 principal sintering processes? Master the Key Methods for Dense, Strong Materials



















