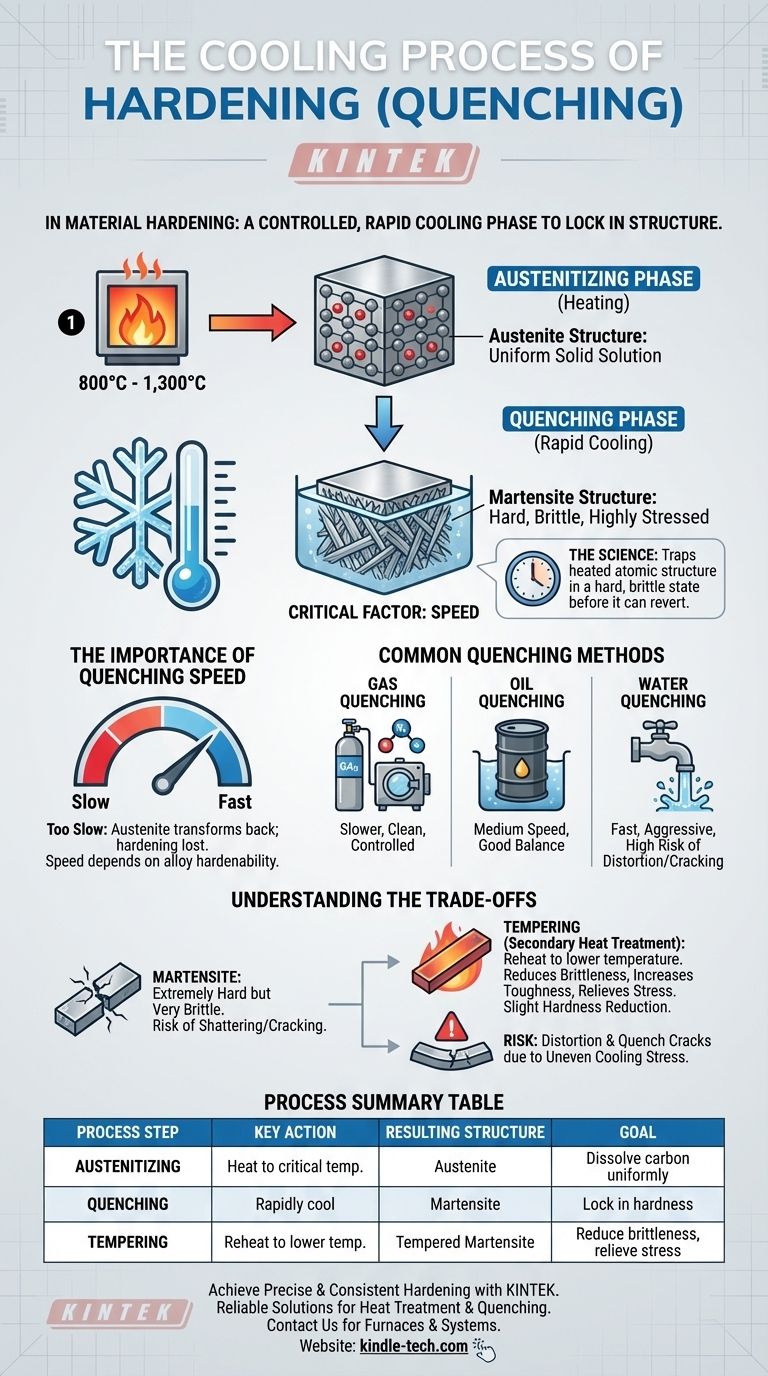
In the context of material hardening, the cooling process is a controlled, rapid cooling phase known as quenching. After heating steel to a specific transformation temperature, it is cooled at a high speed to lock in a new internal structure. This speed is the critical factor that prevents the metal from returning to its softer state, thereby creating a significant increase in hardness and wear resistance.
The goal of quenching is not simply to cool the metal, but to cool it so rapidly that its heated atomic structure is trapped in a hard, brittle, and highly stressed state. This controlled transformation is the entire basis for how hardening works.

The Science Behind Hardening: From Heat to Structure
To understand quenching, you must first understand what happens before it. The entire hardening process is a two-step structural transformation driven by thermal energy.
Step 1: The Austenitizing Phase (Heating)
Before any cooling can occur, the steel must be heated to a specific critical temperature, typically between 800°C and 1,300°C.
Holding the steel at this temperature transforms its internal crystalline lattice into a structure called austenite. In this state, the carbon atoms are uniformly dissolved within the iron, creating a uniform, solid solution.
Step 2: The Critical Cooling Phase (Quenching)
This is the core of the hardening process. The steel, now in its austenitic state, is cooled rapidly.
The rapid temperature drop does not give the carbon atoms time to move out and form the softer structures that exist at room temperature. Instead, the structure is trapped in a new, highly strained, and very hard crystalline form known as martensite.
The Importance of Quenching Speed
The rate of cooling is the single most important variable. If the steel cools too slowly, the austenite will transform back into softer, more stable structures, and the hardening effect will be lost.
The required speed depends on the specific steel alloy. Different alloys have different "hardenability," which dictates how fast they must be quenched to achieve full hardness.
Common Quenching Methods
The medium used for quenching controls the cooling rate. The most common methods include:
- Gas Quenching: Using high-pressure inert gases like nitrogen or argon, often within a vacuum furnace. This provides a clean, controlled, but generally slower quench.
- Oil Quenching: Submerging the part in oil provides a faster quench than gas but slower than water, offering a good balance for many alloys.
- Water Quenching: Provides a very fast and aggressive quench, but the high thermal shock increases the risk of distortion or cracking in some steels.
Understanding the Trade-offs
Hardening is not a "free lunch" in material science. The immense gains in hardness come with significant compromises that must be managed.
Hardness vs. Brittleness
The martensitic structure created by quenching is extremely hard, but it is also very brittle. An as-quenched part can be as fragile as glass and may shatter if subjected to impact.
This is the primary trade-off: you are exchanging the material's ductility and toughness for exceptional hardness.
The Role of Tempering
Because as-quenched steel is often too brittle for practical use, a secondary heat treatment called tempering is almost always performed.
Tempering involves reheating the hardened part to a much lower temperature. This process relieves the internal stresses from quenching and reduces brittleness, making the part tougher. This comes at the cost of a slight reduction in peak hardness.
The Risk of Distortion and Cracking
The rapid and often uneven cooling during quenching is a violent process for the material. It creates immense internal stress as different sections of the part shrink at different rates.
This stress can cause the part to warp, distort, or, in severe cases, develop quench cracks, rendering it useless. Proper part design and quench control are essential to mitigate this risk.
Making the Right Choice for Your Goal
The specific cooling process you choose depends entirely on the desired final properties of the component.
- If your primary focus is maximum hardness and wear resistance: A very fast quench is necessary to ensure a fully martensitic structure, but you must plan for a subsequent tempering cycle to reduce extreme brittleness.
- If your primary focus is a balance of toughness and hardness: A less aggressive quench (e.g., oil instead of water) or a higher tempering temperature might be used to sacrifice some hardness for a significant gain in toughness and impact resistance.
- If your primary focus is minimizing distortion in a complex part: A slower, more controlled method like high-pressure gas quenching is often preferred, even if it means you can only use steel alloys with very high hardenability.
Ultimately, mastering the cooling process is about precisely controlling the material's final structural state to achieve a predictable engineering outcome.
Summary Table:
| Process Step | Key Action | Resulting Structure | Goal |
|---|---|---|---|
| Austenitizing | Heat steel to critical temperature | Austenite | Dissolve carbon uniformly |
| Quenching | Rapidly cool the steel | Martensite | Lock in hardness |
| Tempering | Reheat to a lower temperature | Tempered Martensite | Reduce brittleness, relieve stress |
Achieve precise and consistent material hardening results. The quenching process is critical, and the right equipment ensures control over cooling rates to minimize distortion and cracking while achieving the desired hardness. KINTEK specializes in lab equipment and consumables, providing reliable solutions for your laboratory's heat treatment needs. Contact us today to discuss how our furnaces and quenching systems can enhance your material processing capabilities.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vertical Laboratory Tube Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
People Also Ask
- Why is a vacuum environment necessary for sintering ZrC-SiC? Prevent Oxidation and Ensure Phase Purity
- What are the three steps in sintering cycle in powder metallurgy? Master the Heat Treatment Process
- What role does a precision laboratory oven play in the hydrothermal synthesis of copper sulfate nanocrystals?
- How hot can an electric furnace get? A Guide to Temperature Ranges and Applications
- Why is temperature important in casting? Master the Thermal Balance for Defect-Free Parts
- How does sintering temperature affect density? Optimize Your Process for Maximum Material Performance
- What are the different types of annealing furnace? A Guide to Choosing the Right System for Your Needs
- What are the uses of heat treated aluminum alloys? Unlock High-Strength, Lightweight Performance



















