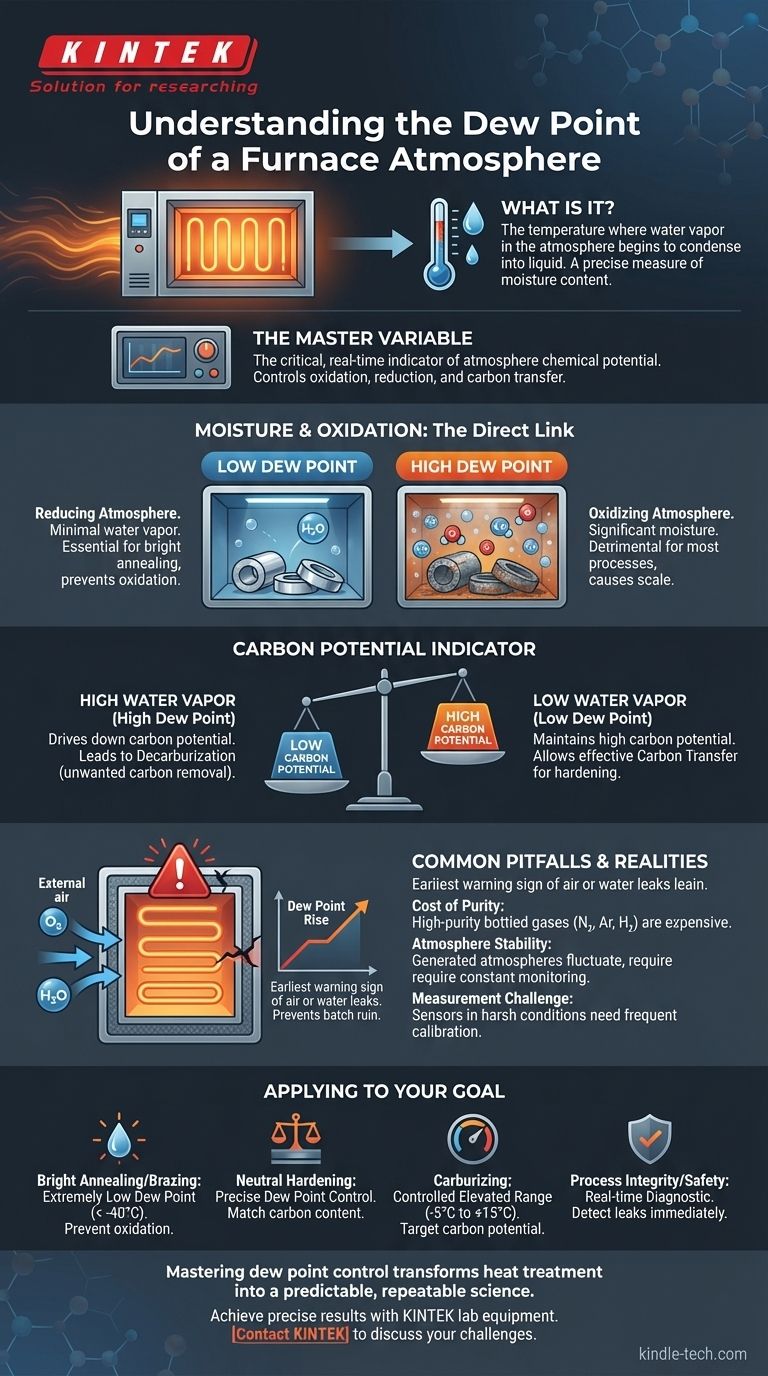
In the context of heat treating, the dew point of a furnace atmosphere is the most practical and precise measure of its moisture content. It is the specific temperature to which the atmosphere gas must be cooled for the water vapor within it to begin condensing into liquid water.
The dew point is not just a measure of water; it is the single most critical, real-time indicator of the atmosphere's chemical potential. Controlling it allows you to directly control the furnace's ability to oxidize, reduce, or transfer carbon to the parts being treated.

Why Dew Point is the Master Variable
While furnace temperature and gas composition are foundational, dew point is the dynamic variable that reveals the true state of the internal environment. It provides immediate feedback on the quality and stability of your process.
The Direct Link Between Moisture and Oxidation
At the high temperatures found in heat treatment, water vapor (H₂O) becomes highly reactive. It dissociates, freeing oxygen atoms that will readily oxidize the surface of most metals, particularly steel.
A low dew point signifies a very dry atmosphere with minimal water vapor. This creates a reducing or non-oxidizing environment, essential for processes like bright annealing where a clean, scale-free surface is required.
A high dew point indicates a wet atmosphere with significant moisture content. This creates a powerfully oxidizing environment, which may be desirable for certain oxide coatings but is detrimental for most hardening and annealing processes.
An Indicator of Carbon Potential
In carbon-controlled atmospheres (like those used for carburizing or neutral hardening), dew point has a direct relationship with carbon potential.
Water vapor reacts with carbon monoxide (CO), a key component in these atmospheres. A higher dew point (more water) will drive down the carbon potential, leading to decarburization—the unwanted removal of carbon from the steel's surface.
Conversely, a lower dew point helps maintain a higher carbon potential, allowing carbon to be effectively transferred into the steel for surface hardening.
The Ultimate Leak Detector
A furnace is designed to be a sealed system, as the references note regarding the importance of seals and chamber integrity. The most common contaminant is external air, which contains both oxygen and moisture.
A sudden or steady rise in the dew point is the clearest and earliest warning sign of an air or water leak into the furnace chamber. This allows operators to identify and correct problems before an entire batch of parts is ruined by oxidation or decarburization.
Common Pitfalls and Practical Realities
Controlling the dew point is essential, but it is not without its challenges. Understanding these trade-offs is key to running an efficient and reliable heat-treating operation.
The Cost of Purity
Achieving an extremely low dew point (e.g., below -50°C) requires high-purity, bottled industrial gases like nitrogen, argon, or hydrogen. While these "inert" gases provide excellent protection, they come at a significantly higher operational cost than atmospheres generated on-site.
Generated vs. Bottled Atmospheres
Atmospheres generated on-site (like endothermic or exothermic gas) are more economical for large-scale operations but are inherently less stable. Their dew point can fluctuate based on the quality of the input air and natural gas, requiring constant monitoring and adjustment.
The Challenge of Measurement
Accurately measuring dew point inside a hot, contaminated furnace environment is difficult. The sensors are exposed to harsh conditions and require frequent calibration and maintenance to provide reliable data. An inaccurate reading can be worse than no reading at all, leading to incorrect process adjustments.
How to Apply This to Your Goal
Your target dew point is entirely dependent on the material you are processing and your desired outcome.
- If your primary focus is bright annealing or brazing: You must maintain an extremely low dew point (typically below -40°C) to prevent any trace of oxidation and ensure a clean, bright finish.
- If your primary focus is neutral hardening of steel: You need to control the dew point to precisely match the carbon content of the steel, preventing both carburization and decarburization.
- If your primary focus is carburizing: You will control the dew point within a specific, elevated range (e.g., -5°C to +15°C) to achieve the target carbon potential for case hardening.
- If your primary focus is process integrity and safety: Use dew point monitoring as a real-time diagnostic tool to immediately detect air leaks that compromise part quality and can create hazardous conditions.
Ultimately, mastering dew point control is what transforms heat treatment from a variable craft into a predictable and repeatable science.
Summary Table:
| Dew Point Significance | Impact on Heat Treatment Process |
|---|---|
| Low Dew Point | Creates a dry, reducing atmosphere; prevents oxidation for bright annealing. |
| High Dew Point | Creates a wet, oxidizing atmosphere; can cause scale and decarburization. |
| Carbon Potential | Directly influences carbon transfer for carburizing or neutral hardening. |
| Leak Detection | A rising dew point is the earliest indicator of an air or water leak. |
Achieve precise, repeatable heat treatment results with expert atmosphere control.
At KINTEK, we understand that mastering your furnace's dew point is essential for preventing oxidation, controlling carbon potential, and ensuring batch integrity. Whether you are bright annealing, carburizing, or hardening, the right lab equipment is key to monitoring and maintaining your atmosphere.
We specialize in providing reliable lab equipment and consumables to meet the exacting demands of laboratory heat treating processes. Let our expertise help you transform your process into a predictable science.
Contact KINTEK today to discuss your specific furnace atmosphere challenges and discover the solutions we can provide.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- Why is a controlled atmosphere furnace with a quartz tube used for W-SiC thin films? Optimize Phase Transformation
- What role does a high-temperature annealing furnace play in regulating the properties of Cerium Oxide nanoparticles?
- How does argon remove oxygen? By Physically Displacing It to Create an Inert Shield
- How does an atmosphere sintering furnace using reducing gases facilitate the formation of AuPd solid solutions?
- What are the primary functions of a high-temperature atmosphere muffle furnace in Fischer-Tropsch Synthesis?
- How does a hydrogen furnace work? Master High-Purity, Oxide-Free Heat Treatment
- Why is nitrogen used in furnaces? Key Benefits for High-Temperature Processes
- Why is an atmosphere-controlled reduction experimental device required? Precision in Ore Pellet Swelling Analysis



















