In short, the primary disadvantage of quenching a part rapidly in water is the extremely high risk of cracking and distortion. The intense thermal shock created by the rapid cooling generates massive internal stresses that can exceed the material's strength, causing the part to warp, twist, or fracture, rendering it unusable.
Water quenching represents a classic engineering trade-off. While it provides the fastest cooling rate to achieve maximum hardness in certain steels, it does so at the expense of part integrity, introducing severe internal stress that often leads to catastrophic failure.

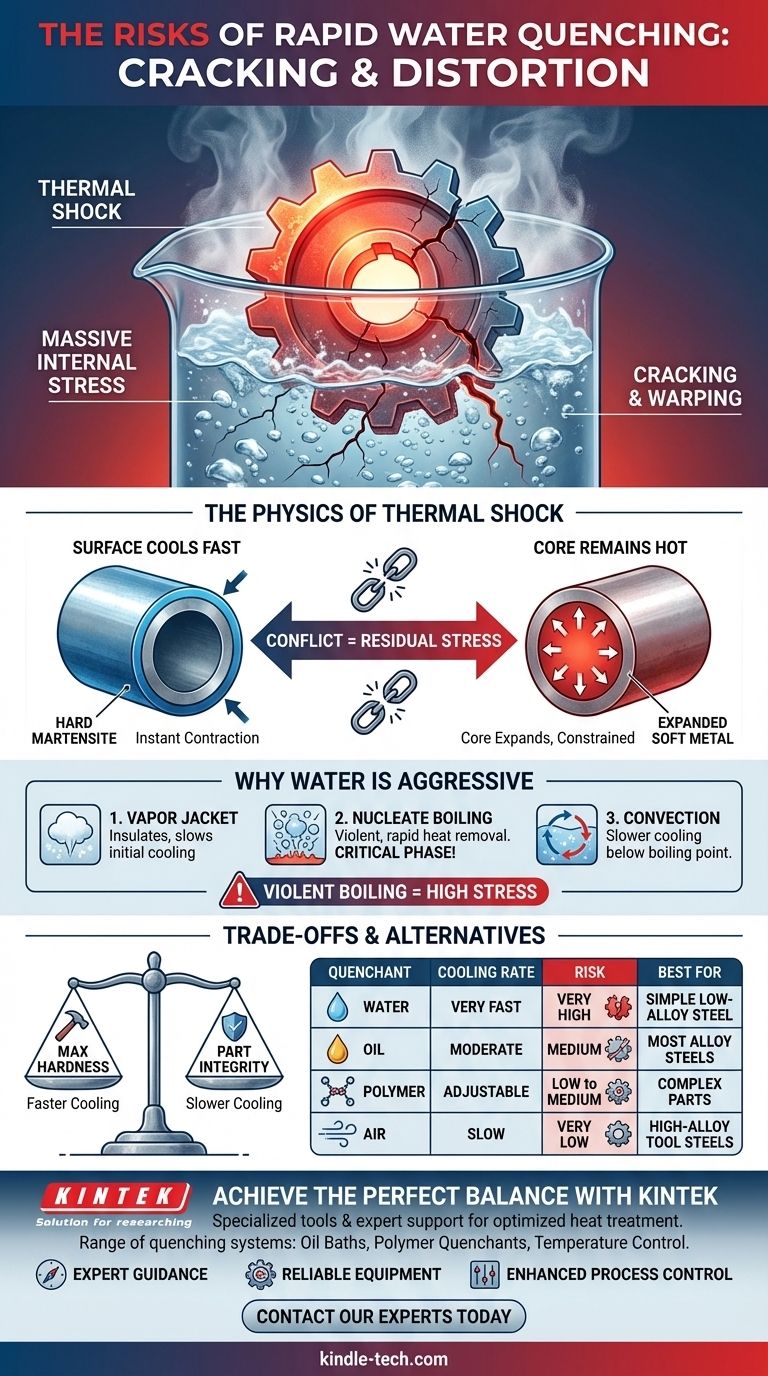
The Physics of Thermal Shock During a Water Quench
To understand why water is so risky, we need to look at what happens at the microscopic level when a hot steel part is submerged. The process is violent and introduces immense stress into the material.
The Problem of Differential Cooling
When you plunge a red-hot steel part into water, the surface cools almost instantaneously. This causes the outer layer to contract and transform into a hard, brittle structure known as martensite.
However, the core of the part is still hot and expanded. This creates a massive temperature gradient between the surface and the center.
How Internal Stresses Build
This conflict—a shrunken, rigid exterior constraining an expanded, hot interior—is the source of residual stress. As the core eventually cools, it too tries to contract, but it is now being pulled on by the already-hardened outer shell.
If these internal pulling and pushing forces become greater than the material's tensile strength, the part will fail. This failure manifests in two ways: distortion (warping) or outright cracking.
Why Water is a Particularly Aggressive Quenchant
Water's high heat capacity and boiling behavior make it an extremely effective—and therefore aggressive—quenching medium. The cooling process occurs in three stages:
- Vapor Jacket: A film of steam initially surrounds the part, which insulates it and slows cooling.
- Nucleate Boiling: This jacket violently collapses, and bubbles form and are rapidly swept away, pulling heat from the surface at a tremendous rate. This is the most aggressive cooling phase.
- Convection: Once the part cools below water's boiling point, cooling slows down and is driven by simple liquid convection.
This violent boiling stage is what makes water quenching so severe compared to slower media like oil.
Understanding the Trade-offs: Hardness vs. Integrity
The decision to use water is a calculated risk. You are trading the integrity of the component for the highest possible hardness.
The Benefit: Achieving Maximum Hardness
The goal of quenching is to cool steel fast enough to prevent the formation of softer microstructures and force the creation of hard martensite.
For simple, low-alloy steels (like 1045 or 1095 carbon steel), a water quench is often the only way to achieve the necessary cooling rate for full hardness. Slower quenchants like oil simply can't remove heat fast enough.
The Risk: Distortion and Cracking
The primary risk is part failure. Cracks often originate at sharp internal corners, keyways, or drastic changes in cross-section, as these features act as stress concentrators.
Distortion is also a major concern, as a warped part may not meet dimensional tolerances, making it useless even if it doesn't crack.
Factors That Increase the Risk
Not all parts are equally susceptible. The risk of cracking when water quenching increases significantly with:
- Part Complexity: Intricate shapes are far more likely to crack than simple, uniform ones.
- High Carbon Content: Steels with higher carbon content form a more brittle martensite, making them more prone to cracking.
- Alloy Content: High-alloy steels (like 4140 or 4340) are designed to harden with a slower quench. Using water on them is unnecessary and extremely risky.
Exploring Safer Quenching Alternatives
If the risk of cracking is too high, several other options provide a more controlled cool-down. The key is to match the quench medium to the steel's hardenability—its ability to form martensite.
Oil Quenching: The Balanced Approach
Oil provides a much slower cooling rate than water. This significantly reduces thermal shock and the risk of distortion and cracking. It is the go-to choice for most common alloy steels that have sufficient hardenability.
Polymer Quenchants: The Tunable Solution
Water-based polymer quenchants offer a major advantage: variable cooling rates. By changing the concentration of polymer in the water, you can engineer a cooling speed that sits somewhere between water and oil, providing a highly controlled and repeatable process.
Air Hardening: For Maximum Stability
High-alloy tool steels (like A2 or D2) are known as "air-hardening" steels. Their chemistry is designed so that they can transform to martensite just by cooling in still or forced air. This is the gentlest quench possible, resulting in minimal stress and excellent dimensional stability.
Selecting the Right Quench for Your Application
Choosing the correct quenching method is about managing risk while achieving the desired metallurgical properties. There is no single "best" quenchant; there is only the right one for the job.
- If your primary focus is maximum hardness on a simple, low-alloy steel: Water quenching is a viable, though risky, option that requires careful process control.
- If your primary focus is dimensional stability and part integrity: Select a steel with higher hardenability and use a slower quenchant like oil or even air.
- If you need a balance of good hardness and process control for complex parts: Polymer quenchants offer the most flexible and reliable solution.
Choosing the right quench is not about finding the fastest cool, but the smartest cool for your specific material, geometry, and engineering goals.
Summary Table:
| Quenching Medium | Cooling Rate | Risk of Cracking | Best For |
|---|---|---|---|
| Water | Very Fast | Very High | Simple, low-alloy steels requiring max hardness |
| Oil | Moderate | Medium | Most alloy steels, balanced approach |
| Polymer | Adjustable | Low to Medium | Complex parts, tunable process |
| Air | Slow | Very Low | High-alloy, air-hardening steels |
Achieve the perfect balance of hardness and part integrity with KINTEK.
Choosing the right quenching process is critical to avoiding costly failures like cracking and distortion. KINTEK specializes in lab equipment and consumables, providing the precise tools and expert support you need to optimize your heat treatment processes.
Whether you're working with simple carbon steels or complex high-alloy components, our range of quenching systems and consumables—including oil baths, polymer quenchants, and temperature control units—ensures you can achieve the desired metallurgical properties without compromising part quality.
Let KINTEK be your partner in precision:
- Expert Guidance: Get recommendations tailored to your specific material and geometry.
- Reliable Equipment: Ensure consistent, repeatable results with our high-quality lab systems.
- Enhanced Process Control: Minimize risk and maximize yield with the right quenching solution.
Contact our heat treatment experts today to discuss how we can help you select the ideal quenching method for your laboratory's needs.
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
People Also Ask
- What can I use a vacuum pump for? Powering Industrial Processes from Packaging to Automation
- What is the purpose of the compression chamber in a vacuum pump? The Heart of Vacuum Generation
- What types of gases can a water circulating vacuum pump handle? Safely Manage Flammable, Condensable & Dirty Gases
- What determines the vacuum degree achievable by a water circulating vacuum pump? Unlock the Physics of Its Limits
- How does a water circulating vacuum pump operate? Discover the Efficient Liquid Piston Principle



















