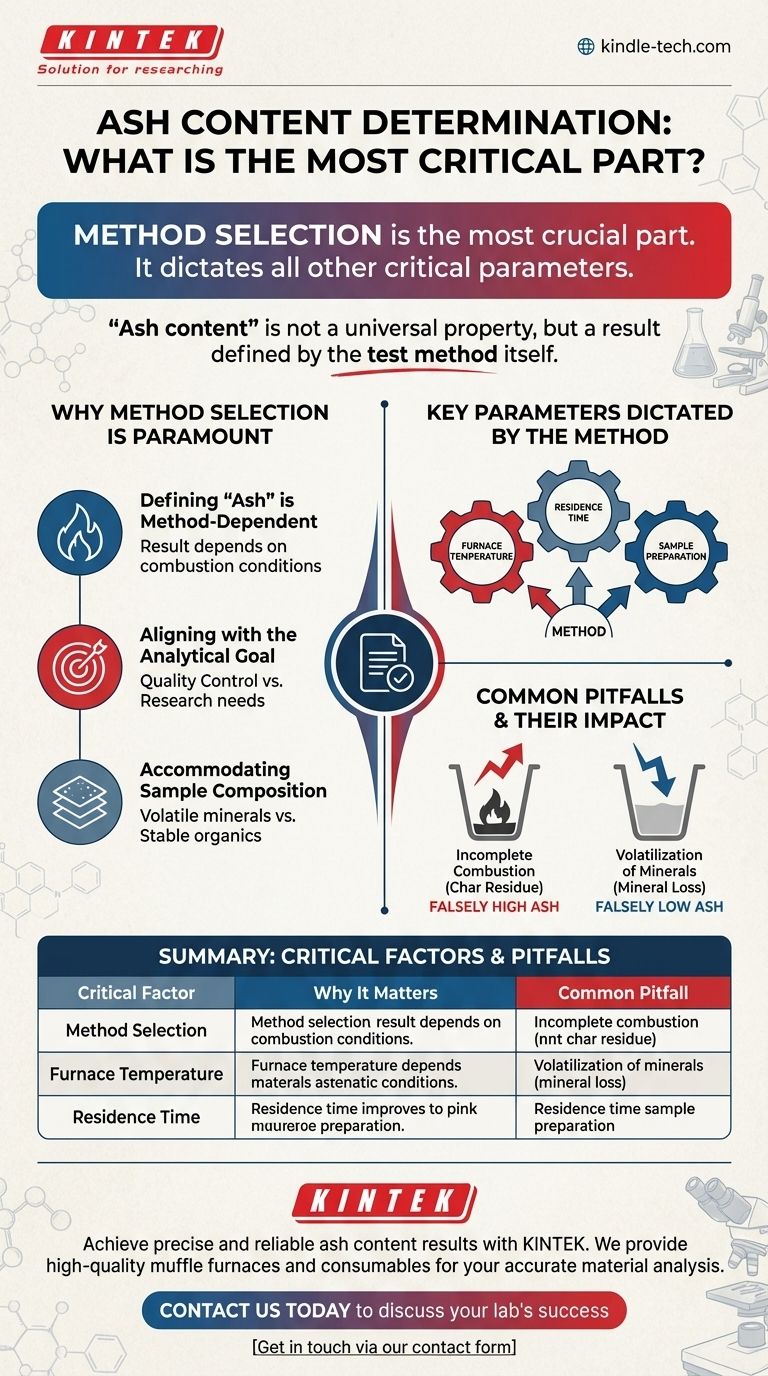
The most crucial part of ash content determination is the selection and consistent application of the correct analytical method for your specific sample and goal. This foundational choice dictates all other critical parameters, such as furnace temperature and heating time, which are essential for producing accurate, repeatable, and meaningful results.
The core takeaway is that "ash content" is not a universal property of a material, but rather a result that is defined by the test method itself. The most significant errors arise not from minor operational mistakes, but from applying a method that is fundamentally unsuited to the material being analyzed or the question being asked.

Why Method Selection is Paramount
The value of an ash content test hinges entirely on using a procedure that is appropriate for the material and the intended purpose of the measurement. A method that works perfectly for one sample type can produce completely invalid data for another.
Defining "Ash" is Method-Dependent
The term "ash" refers to the inorganic, non-combustible residue left after a sample is completely burned. However, the exact chemical composition of this residue depends on the conditions of the combustion process. Different temperatures and durations can change which elements remain and in what form (e.g., oxides, carbonates, sulfates).
Aligning with the Analytical Goal
The reason for the test dictates the method. If you are performing a quality control check on a plastic, you need a standardized method to ensure your results are comparable to a specification. If you are analyzing a food product for total mineral content, you need a different method designed to preserve those minerals without loss.
Accommodating Sample Composition
Different materials behave differently at high temperatures. A method designed for a stable inorganic sample will fail if applied to a material with volatile mineral salts, as those minerals could be lost during heating, leading to an inaccurate result.
Key Parameters Dictated by the Method
Once a method is chosen, it provides a precise blueprint for several critical variables. Consistency in these parameters is non-negotiable for reliable results.
Furnace Temperature
This is often the most significant variable. Too low a temperature will result in incomplete combustion of the organic material, leaving behind carbon residue and artificially inflating the ash weight. Too high a temperature can cause certain inorganic components to decompose or vaporize, leading to a falsely low result.
Residence Time
This refers to how long the sample is heated in the furnace. Insufficient time leads to incomplete combustion, which is a common source of error. The required time is determined by the sample size, its composition, and the furnace temperature.
Sample Preparation
An analysis is only as good as the sample it represents. Proper preparation ensures the small portion being tested is representative of the whole. This often involves grinding, mixing, and drying the sample to create a homogenous material before weighing it for analysis.
Understanding the Common Pitfalls
Even with a chosen method, awareness of potential errors is crucial for interpreting results correctly. The method is designed to mitigate these issues, but they represent the fundamental challenges of the analysis.
Incomplete Combustion
This is the most common error and leads to a falsely high ash content. It occurs when the temperature is too low, the heating time is too short, or the sample is too large. The resulting residue contains unburnt organic material (char) in addition to the true mineral ash.
Volatilization of Minerals
This error leads to a falsely low ash content. At excessively high temperatures, certain mineral salts (like chlorides and carbonates) can decompose and escape as gasses. A properly selected method uses a temperature that is high enough for complete combustion but low enough to prevent this loss.
Making the Right Choice for Your Goal
To ensure your ash content determination is successful, align your approach with your primary objective.
- If your primary focus is ensuring product consistency and quality control: Your priority is the strict adherence to a standardized method (e.g., ASTM, ISO), which guarantees your results are repeatable and comparable.
- If your primary focus is determining the true total mineral content for research: You must carefully select a method that ensures complete combustion without causing volatilization of the specific minerals present in your sample.
Ultimately, a reliable ash content value is the direct result of a deliberately chosen and meticulously executed analytical method.
Summary Table:
| Critical Factor | Why It Matters | Common Pitfall |
|---|---|---|
| Method Selection | Defines what 'ash' is for your sample and goal. | Using an inappropriate method yields invalid data. |
| Furnace Temperature | Ensures complete combustion without mineral loss. | Too low = incomplete combustion; Too high = volatilization. |
| Residence Time | Allows for complete burning of organic material. | Insufficient time leads to char residue (false high ash). |
Achieve precise and reliable ash content results with KINTEK.
Choosing and consistently applying the right method is complex. KINTEK specializes in providing the high-quality lab equipment, including precise muffle furnaces and consumables, that your laboratory needs for accurate material analysis. Our experts can help you select the right tools for your specific application, ensuring your quality control and research goals are met.
Contact us today to discuss how we can support your lab's success and ensure your analytical results are always meaningful. Get in touch via our contact form
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the precautions of muffle furnace? Essential Safety Protocols for Your Lab
- What is the difference between a box furnace and a muffle furnace? Choose the Right Lab Furnace for Your Application
- What is a laboratory furnace called? A Guide to Muffle and Tube Furnaces
- What is the theory of muffle furnace? Achieve Pure, Controlled High-Temperature Processing
- What is the principle working and use of muffle furnace? Achieve Precise, Contamination-Free Heating



















