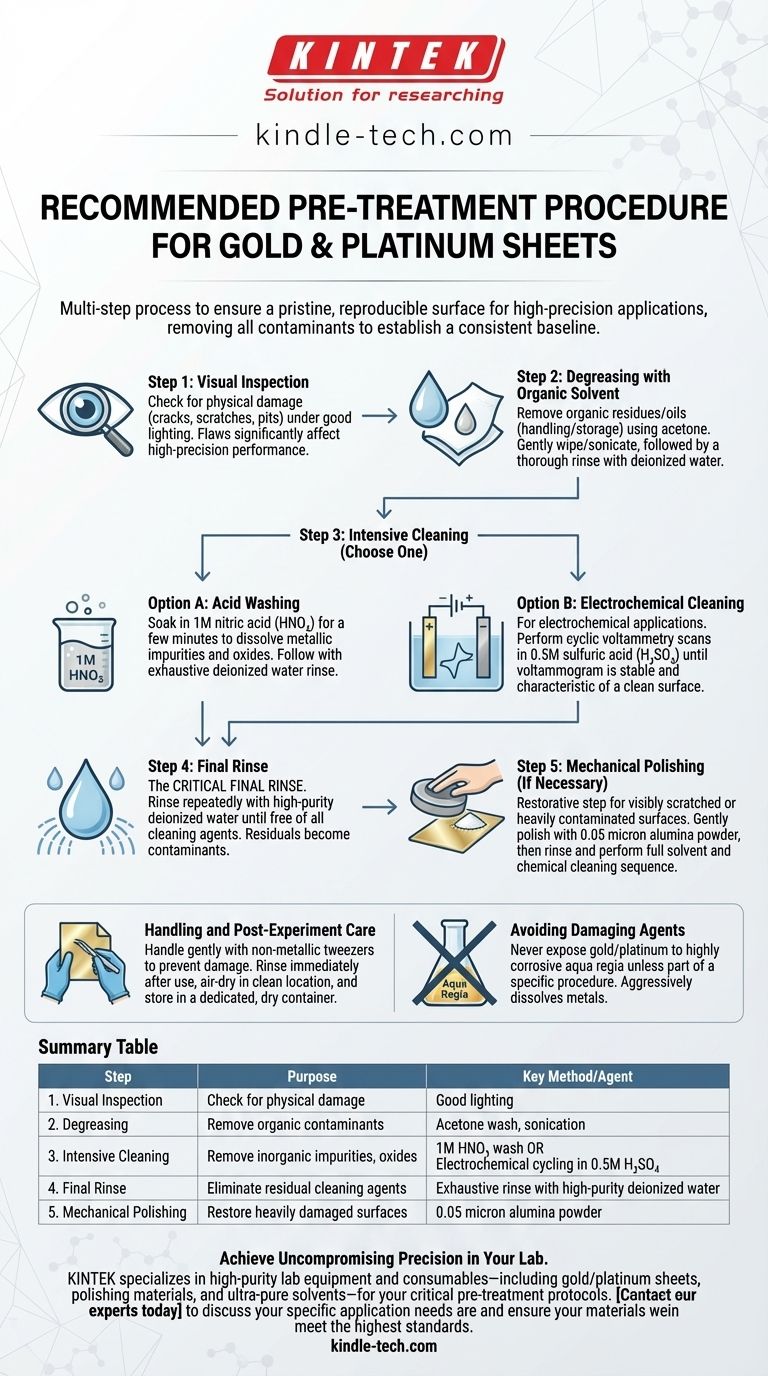
The recommended pre-treatment for gold or platinum sheets is a multi-step process designed to ensure a pristine, reproducible surface for high-precision applications. This procedure begins with a visual inspection and a solvent wash to remove organic contaminants. For more rigorous cleaning, this is followed by either a chemical acid wash or an electrochemical cleaning cycle, concluding with a thorough rinse using high-purity water.
The reliability of your experimental results depends entirely on the initial state of your electrode surface. Effective pre-treatment is not about a single "best" method, but about choosing a consistent cleaning sequence to remove all contaminants and establish a known, active baseline for your measurements.

The Goal: Achieving a Reproducible Surface
Why Pre-Treatment is Non-Negotiable
The surface of a noble metal sheet is where your critical reaction occurs. Any contaminants—such as oils, atmospheric dust, or surface oxides—can block active sites or introduce unwanted side reactions.
This contamination invalidates results by altering the electrode's fundamental behavior. A consistent pre-treatment protocol ensures that every experiment starts from the same clean, well-defined surface.
The Principle of Graduated Cleaning
The most effective approach is to start with the least aggressive cleaning method and escalate only as needed.
This ensures you remove gross contamination (like oils) first before using more intensive methods to address more stubborn impurities or oxides on the metal surface itself.
A Standard Protocol for Pre-Treatment
Step 1: Visual Inspection
Before any cleaning, carefully inspect the sheet's surface under good lighting.
Look for physical damage such as cracks, deep scratches, or pits. Even minor flaws can significantly affect performance in high-precision electrochemical applications.
Step 2: Degreasing with an Organic Solvent
The first cleaning step is to remove organic residues and oils from handling or storage.
Use a suitable organic solvent like acetone. Gently wipe or briefly sonicate the sheet, then immediately and thoroughly rinse it with distilled or deionized water to remove all residual solvent.
Step 3: Intensive Cleaning (Choose One)
After degreasing, a more robust method is required to remove inorganic impurities and surface oxides.
Option A: Acid Washing Soak the sheet in a dilute acid, such as 1M nitric acid (HNO₃), for a few minutes. This effectively dissolves metallic impurities and oxides without attacking the gold or platinum. Follow this with an exhaustive rinse using deionized water.
Option B: Electrochemical Cleaning For electrochemical applications, this is often the preferred method. Place the sheet in a clean electrochemical cell with a non-reactive electrolyte, like 0.5M sulfuric acid (H₂SO₄).
Perform cyclic voltammetry scans across a suitable potential window. Continue cycling until the voltammogram becomes stable and matches the characteristic profile for clean gold or platinum, indicating the surface is pure and active.
Step 4: Mechanical Polishing (If Necessary)
This is a restorative step, not a routine one. It should only be used if the surface is visibly scratched or heavily contaminated in a way that chemical methods cannot resolve.
Gently polish the surface with a fine alumina polishing powder (e.g., 0.05 micron) on a soft pad. Afterward, rinse away all alumina and perform a full solvent and chemical/electrochemical cleaning sequence to remove any embedded polishing material.
Understanding the Trade-offs
The Critical Final Rinse
No matter which cleaning method you use, the final rinse is paramount. Any residual acid, solvent, or polishing compound left on the surface simply becomes a new contaminant.
Rinse repeatedly with high-purity (distilled or deionized) water until you can be confident the surface is free of any cleaning agents.
Handling and Post-Experiment Care
These metals are soft and easily damaged. Handle sheets gently, preferably with non-metallic tweezers, to prevent scratches or deformation.
After an experiment, immediately rinse the sheet with deionized water to remove residual electrolyte. Allow it to air-dry in a clean location before storing it in a dedicated, dry container to prevent re-contamination.
Avoiding Damaging Agents
Never expose gold or platinum to highly corrosive substances like aqua regia (a mixture of nitric and hydrochloric acids) unless it is part of a specific and controlled experimental procedure. This mixture aggressively dissolves these metals.
Making the Right Choice for Your Goal
Your choice of pre-treatment should match the sensitivity of your application and the condition of your electrode.
- If your primary focus is routine analysis with a visually clean sheet: A thorough solvent wash with acetone followed by a deionized water rinse is often sufficient.
- If your primary focus is high-precision electrochemical data: You must incorporate acid washing or electrochemical cycling after the initial solvent wash to ensure a fully active and reproducible surface.
- If your sheet is physically scratched or heavily contaminated: Gentle mechanical polishing is necessary, but it must be followed by a full solvent and chemical cleaning sequence to restore a pristine surface.
A consistent and well-documented pre-treatment protocol is the foundation of reliable and trustworthy experimental data.
Summary Table:
| Step | Purpose | Key Method/Agent |
|---|---|---|
| 1. Visual Inspection | Check for physical damage (scratches, pits) | Good lighting |
| 2. Degreasing | Remove organic contaminants (oils, residues) | Acetone wash, sonication |
| 3. Intensive Cleaning | Remove inorganic impurities, surface oxides | 1M HNO₃ acid wash OR Electrochemical cycling in 0.5M H₂SO₄ |
| 4. Final Rinse | Eliminate residual cleaning agents | Exhaustive rinse with high-purity deionized water |
| 5. Mechanical Polishing (if needed) | Restore heavily damaged/contaminated surfaces | 0.05 micron alumina powder |
Achieve Uncompromising Precision in Your Lab
Your research demands surfaces you can trust. KINTEK specializes in the high-purity lab equipment and consumables—including gold and platinum sheets, polishing materials, and ultra-pure solvents—that are essential for executing these critical pre-treatment protocols.
Let us help you build a foundation of reliability for your most sensitive experiments.
Contact our experts today to discuss your specific application needs and ensure your materials meet the highest standards of purity and performance.
Visual Guide
Related Products
- Gold Disc Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
People Also Ask
- What is the proper post-treatment and storage procedure for a gold disc electrode? Ensure Reliable Electrochemical Data
- What is the material and purity of a gold disc electrode? Ensuring Precision in Electrochemical Analysis
- What are the key precautions for a gold disc electrode? Ensure Accurate Results & Long Lifespan
- What is the operating principle of a gold disc electrode in an electrochemical system? Unlock Precision with a Stable Interface
- What is the typical role of a gold disc electrode in an electrochemical setup? Your Guide to a Precise Working Electrode



















